- Antimony(III) acetate
-
Antimony(III) acetate 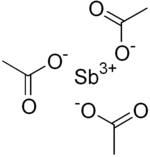 Antimony(III) acetateOther namesAntimony triacetate
Antimony(III) acetateOther namesAntimony triacetate
Acetic acid, antimony(3+) salt
Octan antimonity<Identifiers CAS number 6923-52-0 ChemSpider 21839 
Jmol-3D images Image 1
Image 2- CC(=O)[O-].CC(=O)[O-].CC(=O)[O-].[SbH3+3]
CC(=O)[O-].CC(=O)[O-].CC(=O)[O-].[Sb+3]
- InChI=1S/3C2H4O2.Sb/c3*1-2(3)4;/h3*1H3,(H,3,4);/q;;;+3/p-3

Key: JVLRYPRBKSMEBF-UHFFFAOYSA-K
InChI=1/3C2H4O2.Sb.3H/c3*1-2(3)4;;;;/h3*1H3,(H,3,4);;;;/q;;;+3;;;/p-3/r3C2H4O2.H3Sb/c3*1-2(3)4;/h3*1H3,(H,3,4);1H3/q;;;+3/p-3
Key: NSMVVPJZMRQLMR-ZHOQVWKLAW
InChI=1/3C2H4O2.Sb/c3*1-2(3)4;/h3*1H3,(H,3,4);/q;;;+3/p-3
Key: JVLRYPRBKSMEBF-DFZHHIFOAU
Properties Molecular formula C6H9O6Sb Molar mass 298.89 g mol−1 Appearance White powder Density 1.22 g/cm³ (20 °C) Melting point 127 °C, 400 K, 261 °F
Hazards NFPA 704 LD50 4480 mg/kg (rat)  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Antimony(III) acetate is the antimony salt of acetic acid with the chemical formula of Sb(CH3COO)3. It has the appearance of a white powder and is used as a catalyst in the production of synthetic fibers. It can be prepared by the reaction of antimony(III) oxide with acetic acid:
- Sb2O3 + 6 HC2H3O2 → 2 Sb(C2H3O2)3 + 3H2O
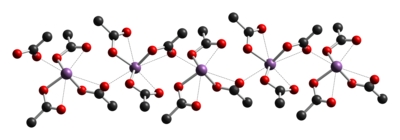
The crystal structure of antimony(III) acetate has been determined by X-ray crystallography. It consists of discrete Sb(OAc)3 monomers with monodentate acetate ligands. The monomers are linked together into chains by weaker C=O···Sb intermolecular interactions.[1]
References
- ^ Hall, M.; Sowerby, D. B. (1980). "Antimony(III) acetate and thioacetate: spectra and crystal structures". J. Chem. Soc., Dalton Trans. (8): 1292–1296. doi:10.1039/DT9800001292.
Antimony compounds Categories:- Inorganic compound stubs
- Antimony compounds
- Acetates
- CC(=O)[O-].CC(=O)[O-].CC(=O)[O-].[SbH3+3]
Wikimedia Foundation. 2010.