- Antimony tetroxide
-
Antimony tetroxide 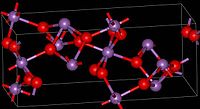 antimony(III,V) oxide
antimony(III,V) oxideIdentifiers CAS number 12786-74-2, 1332-81-6 Properties Molecular formula SbO2; Sb2O4 Molar mass 153.7588; 307.5176 g/mol Appearance white solid Density 6.64 g/cm3 (orthorhombic form) [1] Melting point dec.
Boiling point dec.
Solubility in water insoluble Refractive index (nD) 2.0 Structure Crystal structure orthorhombic Hazards NFPA 704 Related compounds Related compounds Antimony trioxide
Antimony pentoxide tetroxide (verify) (what is:
tetroxide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Antimony tetroxide is an inorganic compound with the formula Sb2O4. This material, which exists as the mineral cervantite,[2] is white but reversibly yellows upon heating. The material, with empirical formula SbO2, is called antimony tetroxide to signify the presence of two kinds of Sb centers.
Formation and structure
The material forms when Sb2O3 is heated in air:[3]
- Sb2O3 + 0.5 O2 → Sb2O4 ΔH = −187 kJ/mol
At 800 °C, antimony(V) oxide loses oxygen to give the same material:
- Sb2O5 → Sb2O4 + 0.5 O2 ΔH = −64 kJ/mol
The material is mixed valence, containing both Sb(V) and Sb(III) centers. Two polymorphs are known, one orthorhombic (shown in the infobox) and one monoclinic.[1] Both forms feature octahedral Sb(V) centers arranged in sheets with distorted Sb(III) centers bound to four oxides.
References
- ^ a b J. Amador, E. Gutierrez Puebla, M. A. Monge, I. Rasines, and C. Ruiz Valero "Diantimony Tetraoxides Revisited" Inorganic Chemistry 1988, Volume 27, pp. 1367–1370. doi:10.1021/ic00281a011
- ^ "Cervantite". Webminerals. http://webmineral.com/data/Cervantite.shtml. Retrieved 2009-06-06.
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
Antimony compounds Categories:- Antimony compounds
- Oxides
- Mixed valence compounds
Wikimedia Foundation. 2010.

