- 1,4-Dioxane
-
Not to be confused with 1,4-Dioxin."Dioxane" redirects here. For other uses, see Dioxane (disambiguation).
1,4-Dioxane 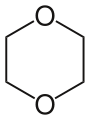
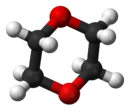 1,4-Dioxane
1,4-Dioxane
1,4-DioxacyclohexaneOther names[1,4]Dioxane
p-Dioxane
[6]-crown-2Identifiers CAS number 123-91-1 
ChemSpider 29015 
UNII J8A3S10O7S 
EC number 204-661-8 DrugBank DB03316 KEGG C14440 
ChEBI CHEBI:47032 
ChEMBL CHEMBL453716 
Jmol-3D images Image 1 - O1CCOCC1
Properties Molecular formula C4H8O2 Molar mass 88.11 g mol−1 Density 1.033 g/mL Melting point 11.8 °C, 285 K, 53 °F
Boiling point 101.1 °C, 374 K, 214 °F
Solubility in water Miscible Thermochemistry Std enthalpy of
formation ΔfHo298-354 kJ/mol Std enthalpy of
combustion ΔcHo298-2363 kJ/mol Standard molar
entropy So298196.6 J·K–1·mol–1 Hazards EU classification Flammable (F)
Carc. Cat. 3
Irritant (Xn)R-phrases R11, R19, R36/37,
R40, R66S-phrases (S2), S9, S16,
S36/37, S46NFPA 704 Flash point 12 °C  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 1,4-Dioxane, often called dioxane because the other isomers of dioxane are rare, is a heterocyclic organic compound. It is a colorless liquid with a faint sweet odor similar to that of diethyl ether. It is classified as an ether. This colorless liquid is mainly used as a stabilizer for the solvent trichloroethane. It is an occasionally used solvent for a variety of practical applications as well as in the laboratory.
Contents
Synthesis and structure
Dioxane is produced by the acid-catalysed dehydration of diethylene glycol, which in turn arises from the hydrolysis of ethylene oxide. The molecule is centrosymmetric, meaning that it adopts a chair conformation, typical of relatives of cyclohexane. The molecule is conformationally flexible, and the boat conformation is easily adopted, as required for chelation to metal cations. In 1985, the global production capacity for dioxane was between 11,000 and 14,000 tons.[1] In 1990, the total U.S. production volume of dioxane was between 10,500,000 and 18,300,000 pounds.[2]
Dioxane has three isomers: in addition to 1,4-dioxane, 1,2-dioxane and 1,3-dioxane are also known.
The three isomers of dioxane. Small blue numbers show numbering of atoms in rings.Uses
Dioxane is primarily used as a stabilizer for 1,1,1-trichloroethane for storage and transport in aluminium containers. Normally aluminium is protected by a passivating oxide layer, but when these layers are disturbed, highly reactive metallic aluminium is exposed to the chlorocarbon. This aluminium reacts with 1,1,1-trichloroethane to give aluminium trichloride, which in turn catalyses the dehydrohalogenation of the remaining 1,1,1-trichloroethane to vinylidene chloride and hydrogen chloride. Reflecting its properties as a ligand, dioxane "poisons" the aluminum trichloride catalyst, by formation of an adduct.[1] Apart from its use as a stabilizer, dioxane is used in a variety of applications as a solvent, e.g. in inks and adhesives.
Solvent properties
Dioxane is relatively nonpolar but has superior dissolving power relative to diethyl ether. Diethyl ether is rather insoluble in water, whereas dioxane is miscible and in fact is hygroscopic. At standard pressure, the mixture of water and dioxane in the ratio 17.9:82.1 by mass is a positive azeotrope that boils at 87.6°C.[3] Dioxane is a versatile polar aprotic solvent. The oxygen atom is Lewis basic, so it is able to solvate many inorganic compounds. Because of its lower toxicity, it is substituted for tetrahydrofuran (THF) in some processes. However, it has a higher boiling point (101 °C versus 66 °C for THF), which is important when reactions are to be conducted at a higher temperature.
The oxygen centres are Lewis basic and so dioxane serves as a chelating diether ligand. It reacts with Grignard reagents to precipitate the magnesium dihalide. In this way, dioxane is used to drive the Schlenk equilibrium.[1] Dimethylmagnesium is prepared in this manner:[4][5]
- 2 CH3MgBr + (C2H4O)2 → MgBr2(C2H4O)2 + (CH3)2Mg
It is used as an internal standard for proton NMR spectroscopy in D2O.[6]
-
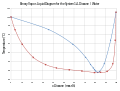
Binary phase diagram for the system 1,4-Dioxane/Water.
Toxicology
Safety
Dioxane is a relatively nontoxic substance with an LD50 of 5170 mg/kg.[1] This compound is irritating to the eyes and respiratory tract. It is suspected of causing damage to the central nervous system, liver and kidneys.[7] Accidental worker exposure to 1,4-dioxane has resulted in several deaths.[8] Dioxane is classified by the IARC as a Group 2B carcinogen: possibly carcinogenic to humans because it is a known carcinogen in animals.[9] The U.S. Environmental Protection Agency classifies dioxane as a probable human carcinogen (having observed an increased incidence of cancer in controlled animal studies, but not in epidemiological studies of workers using the compound), and a known irritant (with a no-observed-adverse-effects level of 400 milligrams per cubic meter) at concentrations significantly higher than those found in commercial products.[10] Under Proposition 65, dioxane is classified in the U.S. state of California to cause cancer.[11] Dioxane is toxic to rats.[12][13][14]
Like some other ethers, dioxane combines with atmospheric oxygen on standing to form explosive peroxides. Distillation of dioxanes concentrates these peroxides increasing the danger.
Environment
Dioxane at the level of 1 μg/L has been detected in many locations in the US.[2] In the State of New Hampshire alone in 2010 it had been found at 67 sites, ranging in concentration from 2 ppb to over 11,000 ppb. Thirty of these sites are solid waste landfills, most of which have been closed for years. It also has low toxicity to aquatic life and can be biodegraded via a number of pathways.[15] Dioxane has affected groundwater supplies in several areas. The problems are exacerbated since dioxane is highly soluble in water, does not readily bind to soils, and readily leaches to groundwater. It is also resistant to naturally occurring biodegradation processes. Due to these properties, a dioxane plume is often much larger (and further downgradient) than the associated solvent plume.
Cosmetics
As a byproduct of the ethoxylation process, a route to some ingredients found in cleansing and moisturizing products, dioxane can contaminate cosmetics and personal care products such as deodorants, shampoos, toothpastes and mouthwashes.[16][17] The ethoxylation process makes the cleansing agents, such as sodium lauryl sulfate, less abrasive and offers enhanced foaming characteristics. 1,4-Dioxane is found in small amounts in some cosmetics, a yet unregulated substance used in cosmetics in both China and the U.S.[18]
In 2008, testing sponsored by the U.S. Organic Consumers Association found dioxane in almost half of tested organic personal-care products.[19] Since 1979 the U.S. Food and Drug Administration (FDA) have conducted tests on cosmetic raw materials and finished products for the levels of 1,4-dioxane.[20] 1,4-Dioxane was present in ethoxylated raw ingredients at levels up to 1410 ppm, and at levels up to 279 ppm in off the shelf cosmetic products.[20] Levels of 1,4-dioxane exceeding 85 ppm in children's shampoos indicate that close monitoring of raw materials and finished products is warranted.[20] While the FDA encourages manufacturers to remove 1,4-dioxane, it is not required by federal law.[21] A number of health authorities around the world have banned imports of products containing 1,4-dioxane.
See also
References
- ^ a b c d Surprenant, Kenneth S. (2000). Dioxane in Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a08_545.
- ^ a b "1, 4-Dioxane Fact Sheet: Support Document". OPPT Chemical Fact Sheets. United States Environmental Protection Agency. February 1995. http://www.epa.gov/chemfact/dioxa-sd.pdf.
- ^ Schneider, C. H.; Lynch, C. C.: The Ternary System: Dioxane-Ethanol-Water in J. Am. Chem. Soc., 1943, vol. 65, pp 1063–1066. doi:10.1021/ja01246a015
- ^ Cope, Arthur C. (1935). Journal of the American Chemical Society 57 (11): 2238. doi:10.1021/ja01314a059.
- ^ Anteunis, M. (1962). "Studies of the Grignard Reaction. II. Kinetics of the Reaction of Dimethylmagnesium with Benzophenone and of Methylmagnesium Bromide-Magnesium Bromide with Pinacolone". The Journal of Organic Chemistry 27 (2): 596. doi:10.1021/jo01049a060.
- ^ Shimizu, A.; Ikeguchi, M.; Sugai, S. (1994). "Appropriateness of DSS and TSP as internal references for 1H NMR studies of molten globule proteins in aqueous media". Journal of Biomolecular NMR 4 (6): 859. doi:10.1007/BF00398414.
- ^ "International Chemical Safety Card". National Institute for Occupational Safety and Health. http://www.cdc.gov/niosh/ipcsneng/neng0041.html. Retrieved 2006-02-02.
- ^ "OPPT Chemical Fact Sheets 1,4-Dioxane (CAS No. 123-91-1)". United States Environmental Protection Agency. http://www.epa.gov/opptintr/chemfact/dioxa-fs.txt. Retrieved 2006-02-02.
- ^ "Eleventh Report on Carcinogens". United States Department of Health and Human Services’ National Toxicology Program. http://ntp-server.niehs.nih.gov/ntp/roc/eleventh/profiles/s080diox.pdf. Retrieved 2006-02-02.
- ^ 1,4-Dioxane (1,4-Diethyleneoxide). Hazard Summary. U.S. Environmental Protection Agency. Created in April 1992; Revised in January 2000. Fact Sheet
- ^ "Proposition 65 List of Chemicals" (PDF). Office of Environmental Health Hazard Assessment. 1,4-Dioxane cancer 123-91-1 January 1988. http://www.oehha.org/prop65/prop65_list/files/P65single040210.pdf.
- ^ Kano, Hirokazu; Umeda, Yumi; Saito, Misae; Senoh, Hideki; Ohbayashi, Hisao; Aiso, Shigetoshi; Yamazaki, Kazunori; Nagano, Kasuke et al. (2008). "Thirteen-week oral toxicity of 1,4-dioxane in rats and mice". The Journal of toxicological sciences 33 (2): 141–53. doi:10.2131/jts.33.141. PMID 18544906.
- ^ Kasai, T; Saito, M; Senoh, H; Umeda, Y; Aiso, S; Ohbayashi, H; Nishizawa, T; Nagano, K et al. (2008). "Thirteen-week inhalation toxicity of 1,4-dioxane in rats". Inhalation toxicology 20 (10): 961–71. doi:10.1080/08958370802105397. PMID 18668411.
- ^ Kasai, T; Kano, H; Umeda, Y; Sasaki, T; Ikawa, N; Nishizawa, T; Nagano, K; Arito, H et al. (2009). "Two-year inhalation study of carcinogenicity and chronic toxicity of 1,4-dioxane in male rats". Inhalation toxicology 21 (11): 889–97. doi:10.1080/08958370802629610. PMID 19681729.
- ^ Kinne, Matthias; Poraj-Kobielska, Marzena; Ralph, Sally A.; Ullrich, René; Hofrichter, Martin; Hammel, Kenneth E. (2009). "Oxidative cleavage of diverse ethers by an extracellular fungal peroxygenase". The Journal of biological chemistry 284 (43): 29343–9. doi:10.1074/jbc.M109.040857. PMC 2785565. PMID 19713216. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2785565.
- ^ Tenth Report on Carcinogens. U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program, December 2002.
- ^ "Chemical Encyclopedia: 1,4-dioxane". Healthy Child Healthy World. http://healthychild.org/issues/chemical-pop/1,4-dioxane/. Retrieved 2009-12-14.
- ^ "Watchdog issues inspection results on Johnson & Johnson". China Daily (Xinhua). March 21, 2009. http://www.chinadaily.com.cn/china/2009-03/21/content_7603271.htm.
- ^ "Carcinogenic 1,4-Dioxane Found in Leading 'Organic' Brand Personal Care Products" (Press release). Organic Consumers Association. March 14, 2008. http://www.organicconsumers.org/bodycare/DioxaneRelease08.cfm. Retrieved October 6, 2010.
- ^ a b c Black, RE; Hurley, FJ; Havery, DC (2001). "Occurrence of 1,4-dioxane in cosmetic raw materials and finished cosmetic products". Journal of AOAC International 84 (3): 666–70. PMID 11417628.
- ^ FDA/CFSAN--Cosmetics Handbook Part 3: Cosmetic Product-Related Regulatory Requirements and Health Hazard Issues. Prohibited Ingredients and other Hazardous Substances: 9. Dioxane
Categories:- IARC Group 2B carcinogens
- Ether solvents
- Dioxanes
Wikimedia Foundation. 2010.