- Ursodiol
-
Ursodiol, also known as ursodeoxycholic acid and the abbreviation UDCA, is one of the secondary bile acids, which are metabolic byproducts of intestinal bacteria.
Ursodeoxycholic acid 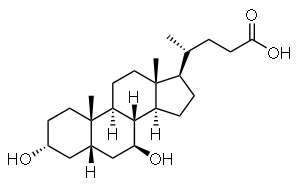
Systematic (IUPAC) name 3α,7β-dihydroxy-5β-cholan-24-oic acid
OR
(R)-4-((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-
10,13-dimethylhexadecahydro-
1H-cyclopenta[a]phenanthren-17-yl)pentanoic acidClinical data Trade names Actigall AHFS/Drugs.com monograph Pregnancy cat. ? Legal status ? Identifiers CAS number 128-13-2 
ATC code A05AA02 PubChem CID 31401 DrugBank DB01586 ChemSpider 29131 
UNII 724L30Y2QR 
KEGG D00734 
ChEBI CHEBI:9907 
ChEMBL CHEMBL1551 
Synonyms ursodeoxycholic acid, Actigall, Ursosan, Urso, Urso Forte Chemical data Formula C24H40O4 Mol. mass 392.56 g/mol SMILES eMolecules & PubChem Physical data Melt. point 203 °C (397 °F)  (what is this?) (verify)
(what is this?) (verify)Contents
Endogenous effects
Primary bile acids are produced by the liver and stored in the gall bladder. When secreted into the colon, primary bile acids can be metabolized into secondary bile acids by intestinal bacteria. Primary and secondary bile acids help the body digest fats. Ursodeoxycholic acid helps regulate cholesterol by reducing the rate at which the intestine absorbs cholesterol molecules while breaking up micelles containing cholesterol. Because of this property, ursodeoxycholic acid is used to treat (cholesterol) gallstones non-surgically.
While some bile acids are known to be colon tumor promoters (e.g. deoxycholic acid), others such as ursodeoxycholic acid are thought to be chemopreventive, perhaps by inducing cellular differentiation and/or cellular senescence in colon epithelial cells.[1]
It is believed to inhibit apoptosis.[2]
Ursodeoxycholic acid has also been shown experimentally to suppress immune response such as immune cell phagocytosis. Prolonged exposure and/or increased quantities of systemic (throughout the body, not just in the digestive system) ursodeoxycholic acid can be toxic.[3]
As a pharmaceutical
Ursodeoxycholic acid goes by the trade names Actigall, Ursosan, Egyurso( Egyphar Egypt), Urso, and Urso Forte. In Italy and Switzerland, it is marketed under the name Deursil. In Mexico it is marketed in capsules of 250 mg under the name Coric by Mexican pharmaceutical Landsteiner Scientific.
Ursodeoxycholic acid can be chemically synthesized and was brought to market by the Montreal-based Axcan Pharma in 1998,[citation needed] which continues to market the drug.
The drug reduces cholesterol absorption and is used to dissolve (cholesterol) gallstones in patients who want an alternative to surgery. The drug is very expensive, however, and if the patient stops taking it, the gallstones tend to recur if the condition that gave rise to their formation does not change. For these reasons, it has not supplanted surgical treatment by cholecystectomy.
It is the only FDA approved drug to treat primary biliary cirrhosis.[4][5]
A Cochrane review to evaluate if ursodeoxycholic acid has any beneficial effect in primary biliary cirrhosis patients included 16 randomized clinical trials with a total of 1447 patients. The primary outcome measures were mortality and mortality or liver transplantation. Although treatment with ursodeoxycholic acid showed a reduction in liver biochemistry, jaundice, and ascites, it did not decrease mortality or liver transplantation.[6]
In absence of biochemical response to 13-15mg/kg/day ursodeoxycholic acid, the 15 year incidence of hepatocellular carcinoma in patients with primary biliary cirrhosis is 20%. [7]
In children, its use is not licensed, as its safety and effectiveness are not established.[8][9][10]
In double the recommended daily dose ursodeoxycholic acid reduces elevated liver enzyme levels in patients with primary sclerosing cholangitis, but its use was associated with an increased risk of serious adverse events (the development of cirrhosis, varices, death or liver transplantation) in patients who received ursodeoxycholic acid compared with those who received placebo). After adjustment for baseline stratification characteristics, the risk was 2.1 times greater for death,transplantation, or minimal listing criteria in patients on ursodeoxycholic acid than for those on placebo (P = 0.038). Serious adverse events, were more common in the ursodeoxycholic acid group than the placebo group (63% versus 37% [P < 0.01])).[11]
Research by the Imperial College London has produced promising results in the treatment of arrhythmia, both in patients who have suffered a heart attack and in foetuses, by using ursodiol to change the electrical properties of myofibroblast cells. Myofibroblasts disrupt the transmission of electrical signals controlling heart rhythm. [12]
Production
The drug is generally not derived from animals. However, it is believed more than 12,000 bile bears are kept on farms in China, Vietnam and South Korea for the purpose of harvesting ursodeoxycholic acid.[13] Ursodeoxycholic acid is found in large quantities in bear bile.
References
- ^ Akare S, Jean-Louis S, Chen W, Wood DJ, Powell AA, Martinez JD (December 2006). "Ursodeoxycholic acid modulates histone acetylation and induces differentiation and senescence". International Journal of Cancer. Journal International Du Cancer 119 (12): 2958–69. doi:10.1002/ijc.22231. PMID 17019713.
- ^ Amaral JD, Viana RJ, Ramalho RM, Steer CJ, Rodrigues CM (September 2009). "Bile acids: regulation of apoptosis by ursodeoxycholic acid". Journal of Lipid Research 50 (9): 1721–34. doi:10.1194/jlr.R900011-JLR200. PMC 2724780. PMID 19417220. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2724780.
- ^ Material Safety Data Sheet on Ursodiol MSDS. https://fscimage.fishersci.com/msds/70916.htm
- ^ Smith T, Befeler AS (March 2007). "High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis". Current Gastroenterology Reports 9 (1): 54–9. doi:10.1007/s11894-008-0021-z. PMID 17335678.
- ^ Jackson H, Solaymani-Dodaran M, Card TR, Aithal GP, Logan R, West J (October 2007). "Influence of ursodeoxycholic acid on the mortality and malignancy associated with primary biliary cirrhosis: a population-based cohort study". Hepatology 46 (4): 1131–7. doi:10.1002/hep.21795. PMID 17685473.
- ^ Gong Y, Huang ZB, Christensen E, Gluud C (2008). Gong, Yan. ed. "Ursodeoxycholic acid for primary biliary cirrhosis". Cochrane Database of Systematic Reviews (3): CD000551. doi:10.1002/14651858.CD000551.pub2. PMID 18677775.
- ^ Kuiper EM, Hansen BE, Adang RP, van Nieuwkerk CM, Timmer R, Drenth JP, Spoelstra P, Brouwer HT, Kuyvenhoven JP, van Buuren HR; Dutch PBC Study Group. Relatively high risk for hepatocellular carcinoma in patients with primary biliary cirrhosis not responding to ursodeoxycholic acid. Eur J Gastroenterol Hepatol. 2010 Dec;22(12):1495-502. PubMed PMID: 21389798.
- ^ Kotb MA (July 2008). "Review of historical cohort: ursodeoxycholic acid in extrahepatic biliary atresia". Journal of Pediatric Surgery 43 (7): 1321–7. doi:10.1016/j.jpedsurg.2007.11.043. PMID 18639689.
- ^ Paediatric Formulary Committee (2008). British National Formulary for Children 2008. London: Pharmaceutical Press. p. 91. ISBN 0-85369-780-9.
- ^ Urso package insert. Birmingham, AL: Axcan Pharma U.S.; 2000 Jan.http://www.axcan.com/pdf/urso_patient_brochure.pdf
- ^ Lindor KD, Kowdley KV, Luketic VA, et al. (September 2009). "High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis". Hepatology 50 (3): 808–14. doi:10.1002/hep.23082. PMC 2758780. PMID 19585548. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2758780.
- ^ BBC News
- ^ Richard Black (11 June 2007). "BBC Test kit targets cruel bear trade". BBC News. http://news.bbc.co.uk/2/hi/science/nature/6742671.stm.
External links
Bile and liver therapy (A05) Bile therapy bile acid (Chenodeoxycholic acid, Cholic acid, Ursodeoxycholic acid) • Nicotinyl methylamide • Piprozolin • Hymecromone • CyclobutyrolLiver therapy Arginine glutamate • Silymarin • Citiolone • Epomediol • Ornithine oxoglutarate • Tidiacic arginine • GlycyrrhizinCategories:- Drugs
- Bile acids
Wikimedia Foundation. 2010.
