- Mechanotransduction
-
Mechanotransduction refers to the many mechanisms by which cells convert mechanical stimulus into chemical activity.[1][2]
Contents
Tendon
The process of mechanotransduction is explained for the lay-reader at http://bjsm.bmj.com/content/43/4/247.full
Ear
One such mechanism is the opening of ion channels in the hair cells of the cochlea in the inner ear.
Air pressure changes in the ear canal cause the vibrations of the tympanic membrane and middle ear ossicles. At the end of the ossicular chain, movement of the stapes footplate within the oval window of the cochlea, in turn, generates a pressure field within the cochlear fluids, imparting a pressure differential across the basilar membrane. A sinusoidal pressure wave results in localized vibrations of the organ of Corti: near the base for high frequencies, near the apex for low frequencies. The cochlea thus acts as an “acoustic prism”, distributing the energy of each Fourier component of a complex sound at different locations along its longitudinal axis. Hair cells in the cochlea are stimulated when the basilar membrane is driven up and down by differences in the fluid pressure between the scala vestibuli and scala tympani. Because this motion is accompanied by a shearing motion between the tectorial membrane and the reticular lamina of the organ of Corti, the hair bundles that link the two are deflected, which initiates mechano-electrical transduction. When the basilar membrane is driven upward, shear between the hair cells and the tectorial membrane deflects hair bundles in the excitatory direction, toward their tall edge. At the midpoint of an oscillation the hair bundles resume their resting position. When the basilar membrane moves downward, the hair bundles are driven in the inhibitory direction.
Basilar Membrane motion causes a shearing motion between the reticular lamina and the tectorial membrane, thereby activating the mechano-sensory apparatus of the hair bundle, which in turn generates a receptor potential in the hair cells.
Thus the sound pressure wave is transduced to an electrical signal which can be processed as sound in higher parts of the auditory system.
Skeletal Muscle
When a deformation is imposed on a muscle, changes in cellular and molecular conformations link the mechanical forces with biochemical signals, and the close integration of mechanical signals with electrical, metabolic, and hormonal signaling may disguise the aspect of the response that is specific to the mechanical forces.[3]
Cartilage
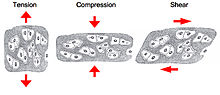
One of the main mechanical functions of articular cartilage is to act as a low-friction, load-bearing surface. Due to its unique location at joint surfaces, articular cartilage experiences a range of static and dynamic forces that include shear, compression and tension. These mechanical loads are absorbed by the cartilage extracellular matrix (ECM), where they are subsequently dissipated and transmitted to chondrocytes (cartilage cells).
Chondrocytes sense and convert the mechanical signals they receive into biochemical signals, which subsequently direct and mediate both anabolic (matrix building) and catabolic (matrix degrading) processes. These processes include the synthesis of matrix proteins (type II collagen and proteoglycans), proteases, protease inhibitors, transcription factors, cytokines and growth factors.,[4][5]
The balance that is struck between anabolic and catabolic processes is strongly influenced by the type of loading that cartilage experiences. High strain rates (such as which occurs during impact loading) cause tissue damage, degradation, decreased matrix production and apoptosis.,[6][7] Decreased mechanical loading over long periods, such as during extended bed-rest, causes a loss of matrix production.[8] Static loads have been shown to be detrimental to biosynthesis [9] while oscillatory loads at low frequencies (similar that of a normal walking gait) have been shown to be beneficial in maintaining health and increasing matrix synthesis.[10] Due to the complexity of in-vivo loading conditions and the interplay of other mechanical and biochemical factors, the question of what an optimal loading regimen may be or whether one exists remain unanswered.
Although studies have shown that, like most biological tissues, cartilage is capable of mechanotransduction, the precise mechanisms by which this is done remain unknown. However, there exist a few hypotheses which begin with the identification of mechanoreceptors.
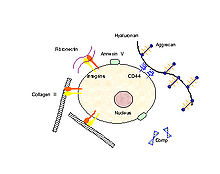
In order for mechanical signals to be sensed, there need to be mechanoreceptors on the surface of chondrocytes. Candidates for chondrocyte mechanoreceptors include stretch-activated ion channels (SAC),[11] the hyaluronan receptor CD44, annexin V (a collagen type II receptor),[12] and integrin receptors (of which there exist several types on chondrocytes).
Using the integrin-linked mechanotransduction pathway as an example (being one of the better studied pathways), it has been shown to mediate chondrocyte adhesion to cartilage surfaces,[13] mediate survival signaling [14] and regulate matrix production and degradation.[15]
Integrin receptors have an extracellular domain that binds to the ECM proteins (collagen, fibronectin, laminin, vitronectin and osteopontin), and a cytoplasmic domain that interacts with intracellular signaling molecules. When an integrin receptor binds to its ECM ligand and is activated, additional integrins cluster around the activated site. In addition, kinases (e.g., focal adhesion kinase, FAK) and adapter proteins (e.g., paxillin, Pax, talin, Tal and Shc) are recruited to this cluster, which is called the focal adhesion complex (FAC). The activation of these FAC molecules in turn, triggers downstream events that up-regulate and /or down-regulate intracellular processes such as transcription factor activation and gene regulation resulting in apoptosis or differentiation.
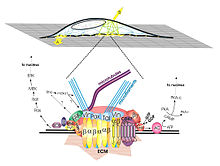 A local force applied to integrins (arrows labeled A and B in top figure) through the extracellular matrix (ECM) activates signal transduction at focal adhesion complex (show as cluster of molecules in bottom figure). This clustering triggers a cascade of reactions that alter the balance of anabolic / catabolic events within the cell.
A local force applied to integrins (arrows labeled A and B in top figure) through the extracellular matrix (ECM) activates signal transduction at focal adhesion complex (show as cluster of molecules in bottom figure). This clustering triggers a cascade of reactions that alter the balance of anabolic / catabolic events within the cell.
In addition to binding to ECM ligands, integrins are also receptive to autocrine and paracrine signals such as growth factors in the TGF-beta family. Chondrocytes have been shown to secrete TGF-b, and upregulate TGF-b receptors in response to mechanical stimulation; this secretion may be a mechanism for autocrine signal amplification within the tissue.[16]
Integrin signaling is just one example of multiple pathways that are activated when cartilage is loaded. Some intracellular processes that have been observed to occur within these pathways include phosphorylation of ERK1/2, p38 MAPK, and SAPK/ERK kinase-1 (SEK-1) of the JNK pathway [17] as well as changes in cAMP levels, actin re-organization and changes in the expression of genes which regulate cartilage ECM content.[18]
More recent studies have hypothesized that chondrocyte primary cilium act as a mechanoreceptor for the cell, transducing forces from the extracellular matrix into the cell. Each chondrocyte has one cilium and it is hypothesized to transmit mechanical signals by way of bending in response to ECM loading. Integrins (α2β1 and α3β1 ) have been identified on the upper shaft of the cilium, acting as anchors to the collagen matrix around it.[19] However, in spite of results from recent studies (such as those from the lab of C.A. Poole) the mechanotransduction role of cilia remains to be proven.
It is important to examine the mechanotransduction pathways in chondrocytes since mechanical loading conditions which represent an excessive or injuruous response upregulates synthetic activity and increases catabolic signalling cascades involving mediators such as NO and MMPs. In addition, studies by Chowdhury TT and Agarwal S have shown that mechanical loading which represents physiological loading conditions will block the production of catabolic mediators (iNOS, COX-2, NO, PGE2) induced by inflammatory cytokines (IL-1) and restore anabolic activities. Thus an improved understanding of the interplay of biomechanics and cell signalling will help to develop therapeutic methods for blocking catabolic components of the mechanotransduction pathway. A better understanding of the optimal levels of in vivo mechanical forces are therefore necessary for maintaining the health and viability of cartilage, preventative techniques may be devised for the prevention of cartilage degradation and disease.
References
- ^ Integrins in Mechanotransduction - Katsumi et al. 279 (13): 12001 - Journal of Biological Chemistry
- ^ Mechanical Strain Induces pp60[IMAGE] Activation and Translocation to Cytoskeleton in Fetal Rat Lung Cells - Liu et al. 271 (12): 7066 - Journal of Biological Chemistry
- ^ Burkholder, Thomas J. (2008). "Mechanotransduction in skeletal muscle". Front Biosci. 12: 174–191.. ISSN PMC2043154. PMC 2043154. PMID 17127292. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2043154.
- ^ Fitzgerald, JB, et al. Mechanical compression of cartilage explants induces multiple time-dependent gene expression patterns and involves intracellular calcium and cyclic AMP. J Biol Chem, 2004. 279(19):p. 19502-11.
- ^ Fitzgerald, JB, et al. Shear and compression differentially regulate clusters of functionally related temporal transcription patterns in cartilage tissue. J Biol Chem, 2006. 281(34): p. 24095-103.
- ^ Kurz, B., et al. Biosynthetic response and mechanical properties of articular cartilage after injurious compression. J Orthop Res, 2001. 19(6): p. 1140-6.
- ^ Loening, AM., et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophy, 2000. 381(2):p.205-12.
- ^ Behrens, F., et al. Biochemical changes in articular cartilage after joint immobilization by casting or external fixation, J Orthop Res, 1989. 7(3): p. 335-43.
- ^ Torzilli, PA., et al. Effect of compressive strain on cell viability in statically loaded articular cartilage. Biomech Model Mechanobiol, 2006. 5(2-3):p.123-32.
- ^ Sah, RLY., et al. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res, 1989. 7(5):p.619-636.
- ^ Mouw, JK., et al. Ion-channel regulation of chondrocyte matrix synthesis in 3D culture under static and dynamic compression. Biomech Model Mechanobiol, 2006.
- ^ von der Mark, K. , et al. Annexin V interactions with collagen. Cell Mol Life Sci, 1997. 53(6): p. 529-45.
- ^ Kurtis, MS., et al. Mechanisms of chondrocyte adhesion to cartilage: role of beta1-integrins, CD44, and annexin V. J Orthop Res, 2001. 19(6): p. 1122-30.
- ^ Pulai, JI., et al. The alpha5beta1 integrin provides matrix survival signals for normal and osteoarthritic human articular chondrocytes in vitro. Arthritis Rheum, 2002. 46(6): p. 1528-35.
- ^ Millward-Sadler, SJ., et al. Mechanotransduction via integrins anad interleukin-4 results in altered aggrecan and matrix metalloproteinase 3 gene expression in normal, but not osteoarthritic, human articular chondrocytes. Arthritis Rheum, 2000. 43(9): p. 2091-9.
- ^ Millward-Sadler, SJ. and Salter, DM. Integrin-dependent signal cascades in chondrocyte mechanotransduction. Ann Biomed Eng, 2004. 32(3): p. 435-46.
- ^ Fanning, PJ., et al. Mechanical regulation of mitogen-activated protein kinase signaling in articular cartilage. J Biol Chem, 2003. 278(51):50940-8.
- ^ Urban, JP. The chondrocyte: a cell under pressure. Br J Rheumatol, 1994. 33(10): p.901-8.
- ^ McGlashan, S. et al. Localization of Extracellular Matrix Receptors on the Chondrocyte Primary Cilium, J Histochemistry and Cytochemistry. 54 (9): 1005-1014, 2006.
- Mammano, F. and R. Nobili, Biophysics of the cochlea: linear approximation. J Acoust Soc Am, 1993. 93(6): p. 3320-32.
- von Bekesy, G., DC resting potential inside the cochlea partition. J Acoust Soc Am, 1952. 24: p. 72-76.
- 1. Kandel, E.R., Schwartz, J.H., Jessell, T.M., Principles of Neural Science. New York: McGraw-Hill ed, ed. 4th. 2000.
- A. J. Hudspeth, Y. Choe, A. D. Mehta, and P. Martin, Putting ion channels to work: Mechanoelectrical transduction, adaptation, and amplification by hair cells, PNAS, October 24, 2000; 97(22): 11765 - 11772.
- A. J. Hudspeth, Nature 341, 397 (1989).
External links
- www.du.edu/~kinnamon/3640/hearing/hearing.html
- MeSH Cellular+Mechanotransduction
- Mechanotransduction Knowledgebase
Signaling pathways Agents Intracellular signaling P+PsSignal transducing adaptor protein: Scaffold protein
2nd messenger: cAMP-dependent pathway · Ca2+ signaling · Lipid signaling · IP3/DAG pathwayBy location Other concepts Signaling molecule · Ion channel gating · Mechanotransduction · Phototransduction · Synaptic transmissionB trdu: iter (nrpl/grfl/cytl/horl), csrc (lgic, enzr, gprc, igsr, intg, nrpr/grfr/cytr), itra (adap, gbpr, mapk), calc, lipd; path (hedp, wntp, tgfp+mapp, notp, jakp, fsap, hipp, tlrp) Categories:
Wikimedia Foundation. 2010.