- Mechanosensitive channels
-
Mechanosensitive channels or mechanosensitive ion channels are membrane proteins capable of responding over a wide dynamic range to external mechanical stimuli. They are found in prokaryotes and eukaryotes. The channels vary in selectivity for the permeating ions from nonselective between anions and cations, to cation selective allowing passage Ca2+, K+ and Na+, a highly selective K+ channels.
All organisms and apparently all cell types sense and respond to a mechanical stimulus.[1] MSCs function as mechanotransducers capable of generating either an electrical and ion flux signal as a response to external stimuli.[2] Under extreme turgor in bacteria, non selective MSCs such as MSCL and MSCS serve as safety valves to prevent lysis. In specialized cells of the higher organisms, other types of MSCs create the senses of hearing and touch and sense the stress needed for muscular coordination. MSCs also allow plants to distinguish up from down due to differences in signal rates resulting from the force of gravity. MSCs are not pressure sensitive, but sensitive to local stress, most likely tension in the surrounding lipid bilayer.[3]
Contents
History
Mechanosensitive channels were first observed in chick skeletal muscles by Falguni Guharay and Frederick Sachs in 1983 and the results were published in 1984 .[4] Since then MS channels have been found in cells from bacteria to human as well as plants.[5] In the last two decades, the knowledge of the structure and function of MSCs has been increased since its discovery. They are present in different kingdoms such as Archaea, Bacteria, Plants, Fungi and Eukarya.[6]
Classification
MS can be classified based on the type of ion to which they are permeable.
Cation Selective MSCs: As the name suggests, they exhibit a selective permeability for positive ions with the most selective channels being those for K+. The most common eukaryotic MSCs are cation selective passing Na+, K+ and Ca+ but not Mg+. They have a single channel conductance range (25-35 pS) and they are blocked by trivalent ion Gadolinium. The K selective MSCs such as TREK-1 are not blocked by Gd+3.[7]
Anion Channels: they exhibit a significant permeability for negative ions, and are not predominant as cation MS. They have a large conductance range (> 300pS).
Non Selective ion channels: As the name indicates, they do not differentiate between positive and negative channels those are more common to Archaea and Bacteria, but rarely found in Eukarya.[8]
Gating Mechanism
Although MS are different in many aspects, structures and function between them, all the MS studies up to date share the important feature gating meaning they all open as a pore-like when protein-channel is activated by a mechanical stimuli. To understand how membrane-activated ion channels open currently there are three models which explained the gating process.
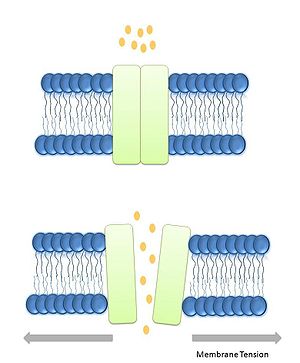
Lipid bilayer Tension or stretch model[9]: In this model tension in the lipid bilayer triggers conformational changes, thus leading to the opening of the channels. The tension perceived by the protein comes from the lipids. It has been demonstrated that the tension/stretch profile in the lipid bilayer is originated by membrane curvature and bilayer-protein hydrophobic mismatch.[10]
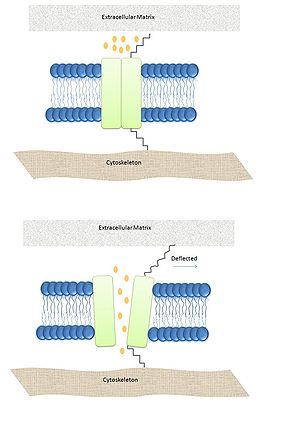
Spring-like Tether model: In this model a spring-like tether is attached directly to the MS channel and can be present in either the cytoskeleton or the extracellular matrix linking these elements together. When external stimuli deflect the tether the displacement opens the channel.[11] This particular mechanism has been demonstrated to be the responsible for gating hair cells which are responsible for hearing in vertebrates.[12]
Bacteria MS
MS channels were first discovered by patch-clamp experiments in E. coli.[13] They have been classified based on their conductance as mini (MscM), small (MscS) and large (MscL). These channels function in tandem-mode and are responsible of turgor regulation in bacteria; when activated by changes in the osmotic pressure. MscM is activated first at really low pressures followed by MscS, and finally MsCL being the last chance of survival during osmotic shock. Their task was demonstrated when bacteria missing both MscS and MscL was lysed after exposure to osmotic downshocks.[14]
MscS: Small conductance mechanosensitive channel.
The main conductance is 1nS in buffer solution. Channel-proteins have been found in gram positive and gram negative bacteria, archaea and plants. MscS channel was found after studies in E. coli spheroplasts10. The identification of the gene family necessitated for MS of small conductance was as two different channels. YggB encoding MscS and KefA encoding MscK in E. coli further confirm its role osmotic regulation. Mutagenesis studies showed that when both genes YggB and KefA were deleted MscS lost its function, but maintain MscL and MscM, but mutants deficient of YggB and MscL showed that the function of those channel is to open in respond to pressure range right before cell rupture.[15]
The 3D structure of this channel at closed state was elucidated after the crystallography study by Bass et al.[16] which showed that at resolution of 3.9 Å this 31kDa protein is an homoheptamer forming a channel with 80 Å of diameter and 120 Å in length, each subunit contains three transmembrane domains (TM1, TM2, and TM3) with the N-terminal facing the periplasm and the C-terminal embedded in the cytoplasm. The TM3 is highly conserved in MscS family and it is thought to play an important role in MS prokaryotic gating.[17] MscS is a small protein composed of 286 amino acid residues activated by both tension in the lipid bilayer and voltage; in 2002 Vasquez et al.[18] detailed this process and showed that during the change from closed state to open state the TM1 tilt and rotate making TM2 being exposed to the membrane and the TM3 helices expand, tilt, and rotate. During the rearrangement the confined part of the pore was measured as 11 Å, and water molecules were more accessible to the TM3. The two transmembrane domains are in continuous contact with the lipid bilayer and are thought to be the sensor for the tension in the lipid bilayer as well as sensor for voltage because of the three arginine residues present in those domains.[19]
Although MscS is activated by voltage it has been demonstrated that, voltage itself is insufficient to open the channel, thus functioning in a cooperative manner with the channel. The more positive voltage, the higher the probabilities of opening the channel as long as pressure over the threshold is still applied in the system; the performance of this channel at higher voltage has not been completely understood. MscS has a small affinity for negative ions including Cl-, and glutamate.[20]
MscL: Large conductance mechanosensitive channel.
In bacteria MscL was the first MS channels cloned and sequenced, and is by far one of the most studied channels. The gene encoding MscL protein is trkA and it is located in the inner membrane of the E. coli. The protein is 17 KDa, and consists of 136 amino acids; mostly hydrophobic residues resulting in two hydrophobic segments, however molecular weight of the functional channel is presumed to be 60-70 KDa from gel filtration experiments, suggesting oligomerization. As a common feature no cysteines residues are present in this channel.[21]
In 1998 the homolog MscL from mycobacterium tuberculosis Tb-MscL was elucidated at closed state by X ray crystallography at 3.5 Å resolution. The protein is a homopentamer composed mostly of helical regions trans orientation of the helices with respect to the bilayer, with two domains: the cytoplasmic and the transmembrane. The channel is 85 Å in length, 35 Å and 50 Å for the cytoplasmic transmembrane domain respectively and 50 Å in diameter. The helices cross the membrane twice with both the C-terminal and the N-terminal, thus having two transmembrane domains TM1 and TM2 being TM1 the most conserved region among MscL proteins especially at the N-terminal region.[22] It is located in the cytoplasm and forms a α-hydrophobic helix called S1; the region between the transmembrane domains form a loop that is divided into two regions: S2 a glycine-proline rich region and S3 a short helical section.[23] Also interestingly the secondary structure of the protein is resistant to thermal denaturation still in the presence of SDS.[24]
During the activation of the prokaryotic MscL by tension in the lipid bilayer an intermediate state was determined. The S1 segments form a bundle when the structure is in the closed state, and the crosslinking of S1 segments prevents the opening of the channel. When tension is applied to the membrane the transmembrane barrel-like structure expand and stretch apart the region S1-TM1 allowing the channel to open.[25] The size of the pore at open state is approximately 25Å. The transition from closed to intermediate state is accompanied by small movements of the TM1; further transitions to the open stated are characterized by big rearrangements in both the TM1 and TM2.[26]
Role of lipid bilayer in MS
The lipid bilayer is an important structure in all living cells; it has many functions such as separation of compartments, and signaling among others. In the case of the prokaryotic protein channels MscS and MscL both are gated by tension in the lipid bilayer, thus suggesting an important role in such a complex structures.
The tension in the membrane bilayer has been extensively studied, simple intrinsic properties of the lipids can account for the contributions in the free energy of the open, intermediate, and close state of the MS channels. The bilayer possess different features that allows it to transducer tension and to prevent exhaustive deformations, the first one is “in plane fluidity of the lipid bilayer” meaning that any in plane tension in the lipid bilayer is felt homogenously in the absence of cytoskeleton interactions. The lipid molecules have a specific space in between them, preventing the bilayer from any changes.[27]
The contribution of membrane deformation in the gating of MS channels can be divided in two types: the deformation of the plane of the bilayer, and the deformation of the thickness of the bilayer. Also during any process involving changes in the structure, the free energy of the process itself is also an important factor. During gating the major processes that account for this event are: hydrophobic mismatch, and membrane curvature. It has been calculated that the free energy of the tension in the lipid bilayer is similar to the energy needed for gating the channels.[28]
A different study showed that the length of the hydrophobic tail affects its functioning as well as supporting the different states, Phosphatidylcholine (PC) 18 stabilizes better the open state of the MscL channel, PC 14 stabilizes the intermediate state, and a mixture of PC 18 and lysophosphatidylcholine (LPC) stabilizes the closed state23, suggesting that the bilayer thickness (for carbon tail lengths of 16, 18 and 20) affects channel function. In conclusion the energy from the environment of the membrane plays an important role in the total energy of channel gating.
Physiological role of MS
MS channels are ubiquitously expressed in the membrane of prokaryotes suggesting their significance. In Bacteria and Archaea the function of these channels is conserved and it has been demonstrated that they play a role in turgor regulation. In Eukarya MS channels are involved in all five senses. The main family is TRP, and one good example is hair cells involved in the hearing process. When a wave of sound deflects the stereocilia, the channel opens. This is an instance of the Spring-like Tether gating mechanism. Recent studies have revealed a new role of mechanosensitive pathways in which naive mesenchymal stem cells are committed to a particular lineage based on the elasticity of its surrounding matrix.[29]
MS have also been suggested as a potential target for antibiotics, the reasoning behind this idea is that both McsS and MscL are highly conserved among prokaryotes, but their homologs have not been found in animals[30] making them an exceptional potential for further studies.
Techniques used to study MS
This is a short list of the most frequently techniques used to study the properties, function, mechanism and other features of these channels.
• Patch-clamp: Single cell recording.
• EPR
• Molecular dynamics simulation: determination of the atomic fluctuation of the system.
• Atomic force Microscopy: mechanical forces of the membrane.
• Micropipette Aspiration: Pressure to cells.
• 3D simulations
• Mutagenesis
MS Diseases
• Polycystic kidney disease.
• Atrial fibrillation
Abnormalities in the function of MS channels can cause5:
I. Neuronal disease
II. Muscular degeneration.
III. cardiac arrhythmias
IV. Hypertension.
References
- ^ 1. Kung C. A possible unifying principle for mechanosensation. Nature 2005; 436:647–54.
- ^ 2. Hackney CM, Furness DN. Mechanotransduction in vertebrate hair cells: structure and function of the stereociliary bundle. AmJ Physiol 1995; 268:C1–138.
- ^ 3. http://www.iop.org/EJ/abstract/1478-3975/1/2/007/
- ^ . PMC 1193237. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1193237.
- ^ 4.http://www.springerlink.com/content/d075747515513ll2/.
- ^ 5. langevin.anu.edu.au/publications/chapter10_martinac.
- ^ 6. http://www.springerlink.com/content/b73288l684890099/.
- ^ 5. Sackin, H. Mechanosensitive channels. Annu. Rev. Physiol. 1995. 57:333-53.
- ^ 6. Markin, V.S., and B. Martinac. 1991. Mechanosensitive ion channels as reporters of bilayer expansion. A theoretical model. Biophys. J. 60:1120–1127.
- ^ 7. Perozo E, Cortes DM, Sompornpisut P, et al. Structure of MscL and the gating mechanism of mechanosensitive channels. Nature 2002; 418:942.
- ^ 8. Lumpkin, E. Caterina M. Mechanisms of sensory transduction in the skin. Nature. 2006: 445:858.
- ^ 9. Hamill, O.P., and D.W. McBride Jr. 1997. Induced membrane hypo /hyper mechanosensitivity A limitation of patch-clamp recording. Ann. Rev. Physiol.59:621–631.
- ^ 10. Martinac B, Buechner M, Delcour AH, Adler J, Kung C: Pressuresensitive ion channel in Escherichia coli. Proc Natl Acad Sci USA 1987, 84:2297-2301.
- ^ 11. Perozo, E., and D.C. Rees. 2003. Structure and mechanism in prokaryotic mecahnosensitive channels. Curr. Opin. Struct. Biol. 13:432–442.
- ^ 12. Levina, N., Totemeyer, S., Stokes, N. R., Louis, P., Jones, M. A., &Booth, I. R. (1999). Protection of Escherichia coli cells againstextreme turgor by activation of MscS and MscL mechanosensitivechannels: Identification of genes required for MscS activity.EMBO J., 18, 1730–1737.
- ^ 13. Bass, R. B., Strop, P., Barclay, M.,&Rees, D. (2002). Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science, 29, 1582–1587.
- ^ 14. Pivetti, C. D., Yen, M. R., Miller, S., Busch,W., Tseng, Y., Booth, I. R. et al. (2003). Two families of mechanosensitive channel proteins. Microbiol. Mol. Biol. Rev., 67, 66–85.
- ^ 15. Vasquez, V., Sotomayor, M., Cordero-Morales, J., Shulten, K., Perozo, E. (2008). A Structural mechanism for MscS gating lipid channels in bilayer. Science, 321, 1210-14.
- ^ 16. F. Bezanilla, E. Perozo, Force and voltage sensors in one structure, Science 298 (2002) 1562–1563.
- ^ 17. Sukharev, S. I., Blount, P., Martinac, B., Kung, C. (1997). MECHANOSENSITIVE CHANNELS OF ESCHERICHIA COLI: The MscL Gene, Protein, and Activities. Annu. Rev. Physiol. 59:633–57
- ^ 18. Sukharev, S. I., Blount, P., Martinac, B., Blattner, F. R., & Kung, C. (1994). A large mechanosensitive channel in E. coli encoded by MscL alone. Nature, 368, 265–268.
- ^ 19. Chang, G., Spencer, R., Barclay, R., Lee, A., Barclay, M., Rees, C. 1998. Structure of the MscL homologue from Mycobacterium tuberculosis: a gated mechanosensitive ion channel, Science. 282, 2220–2226.
- ^ 20. Blount P, Sukharev SI, Moe PC,Schroeder MJ, Guy HR, Kung C. 1996. Membrane topology and multimeric structure of a mechanosensitive channel protein. EMBO J. 15, 18, 4798-4805.
- ^ 21. Arkin IT, Sukharev SI, Blount P, KungC, Br¨unger AT. 1998. Helicity, membrane incorporation, orientation, and thermalstability of the large conductance mechanosensitive ion channel from E.coli. Biochim. Byophys. Acta. 1, 131-140.
- ^ Sukharev,S., Betanzos, M., Chiang, C.S., Guy, H.R. 2001. The gating mechanism of the large mechanosensitive channel MscL, Nature, 409, 720–724.
- ^ 23. Perozo, E., Cortes, D. M., Sompornpisut, P., Kloda, A.,&Martinac, B. (2002). Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature, 418, 942–948.
- ^ 24. Wiggins P, Phillips R (2004) Analytic models for mechanotransduction: Gating a mechanosensitive channel. Proc Natl Acad Sci U S A, 101(12):4071–6
- ^ 25. Wiggins P, Phillips R (2005) Membrane-protein interactions in mechanosensitivechannels. Biophys J, 88(2):880–902.
- ^ 26. Engler, A., Shamik, S., Sweeney, L., Dishe, D. 2006. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 126, 4. 677-689.
- ^ 27. Nguyen, T., Clare, B., & Martinac, B. (2005). The effects of parabens on the mechanosensitive channels. Eur. Biophys. J., 34, 389–396.
28. Tang Y, Cao G, Chen X, et al. A finite element framework for studying the mechanical response of macromolecules: application to the gating of the mechanosensitive channel MscL. Biophys J 2006; 91:1248–63.
29. Perozo E, Kloda A, Cortes DM, et al. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat Struct Biol 2002; 9:696–703.
30. Hamill, O.P., and B. Martinac. 2001. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 81:685–740.
See also
Categories:
Wikimedia Foundation. 2010.