- Myelin oligodendrocyte glycoprotein
-
myelin oligodendrocyte glycoprotein 
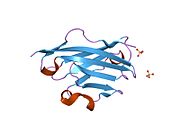
Crystal structure of rat myelin oligodendrocyte glycoprotein.[1]Available structures PDB 1py9 Identifiers Symbols MOG; MGC26137 External IDs OMIM: 159465 MGI: 97435 HomoloGene: 110875 GeneCards: MOG Gene Gene Ontology Molecular function • protein binding
• sodium:dicarboxylate symporter activityCellular component • membrane
• integral to membrane
• plasma membraneBiological process • cell adhesion
• dicarboxylic acid transport
• central nervous system developmentSources: Amigo / QuickGO Orthologs Species Human Mouse Entrez 4340 17441 Ensembl ENSG00000204655 ENSMUSG00000076439 UniProt Q16653 Q61885 RefSeq (mRNA) NM_002433 NM_010814 RefSeq (protein) NP_001008229 NP_034944 Location (UCSC) Chr 6:
29.73 – 29.75 MbChr 17:
37.15 – 37.16 MbPubMed search [1] [2] Myelin Oligodendrocyte Glycoprotein (MOG) is a glycoprotein believed to be important in the process of myelinization of nerves in the central nervous system (CNS). In humans this protein is encoded by the MOG gene.[2][3][4] It is speculated to serve as a necessary “adhesion molecule” to provide structural integrity to the myelin sheath and is known to develop late on the oligodendrocyte.[5]
Contents
Molecular function
While the primary molecular function of MOG is not yet known, its likely role with the myelin sheath is either in sheath “completion and/or maintenance”.[4] More specifically, MOG is speculated to be “necessary” as an "adhesion molecule" on the myelin sheath of the CNS to provide the structural integrity of the myelin sheath.[5]”
MOG’s cDNA coding region in humans have been shown to be “highly homologous”[6] to rats, mice, and bovine, and hence highly conserved. This suggests “an important biological role for this protein”.[4]
Physiology
The gene for MOG, found on chromosome 6p21.3-p22,[7] was first sequenced in 1995[3]. It is a transmembrane protein expressed on the surface of oligodendrocyte cell and on the outermost surface of myelin sheaths. “MOG is a quantitatively minor type I transmembrane protein,[8] and is found exclusively in the CNS. “A single Ig-domain is exposed to the extracellular space[8]” and consequently allows autoantibodies easy access. and therefore easily accessible for autoantibodies.[4]
[8] The MOG “primary nuclear transcript … is 15,561 nucleotides in length[4]” and, for humans, it has eight exons which are “separated by seven introns[4]”. The introns "contain numerous reptitive DNA[4]" sequences, among which is "14 Alu sequences within 3 introns[4]", and have a range varying from 242 to 6484 bp.
Structure
Because of alternatively spliced from human mRNA of MOG gene forming at least nine isoforms. [9]”
The crystal structure of myelin oligodendrocyte glycoprotein was determined by x-ray diffraction at a resolution of 1.45 Angstrom, using protein from the Norway rat. This protein is 139 residues long, and is a member of the immunoglobulin superfamily[1]. The dssp secondary structure of the protein is 6% helical and 43% beta sheet: there are three short helical segments and ten beta strands[10]. The beta strands are within two antiparallel beta sheets that form an immunoglobulin-like beta-sandwich fold[11]. Several features of the protein structure suggest MOG has a role as an "adhesin in the completion and/or compaction of the myelin sheath." There is a "significant strip" of electronegative charge beginning near the N-terminus and running about half the length of the molecule. Also, MOG was shown to dimerize in solution, and the shape complementarity index is high at the dimer interface, suggesting a "biologically relevant MOG dimer."[12]
Synthesis
Developmentally, MOG is formed "very late on oligodendrocytes and the myelin sheath".[5]
Role in disease
Interest in MOG has centered on its role in demyelinating diseases, such as adrenoleukodystrophy, vanishing white matter disease, and multiple sclerosis (MS). It is a target antigen that leads to autoimmune-mediated demyelation. MOG has received much of its laboratory attention in studies dealing with MS. Several studies have shown a role for antibodies against MOG in the pathogenesis of MS.[5]
[13] Animal models of MS, EAE, have shown that “MOG-specific EAE models (of different animal strains) display/mirror human multiple sclerosis[5]”, as is demonstrated by the demyelinating capacity and by the topography of the lesions. These models have shown that the anti-MOG antibodies are the cause of the demyelination. These models with anti-MOG antibodies have been investigated extensively and “are the only antibodies with demyelinating capacity[5]”.
Multiple Sclerosis
The pathogenic process to MS is currently unknown, but there are a couple of theories based on current research. One of the current leading theories is "antibody-mediated demyelination", where the immune system is attacking the body: specifically the central nervous system, leading to demyelination. In this theory, target antigens mark the body for antibodies to attack. Going into more detail, it is the T-cell and B-cells that “have been widely implicated in the MS pathogenesis through an antibody-mediated demylination”.[14] Two suspected antigens involved in pathogenesis of MS are commonly investigated among a large number of various antigens in some of the studies that will be mentioned. One is myelin basic protein (MBP), which has already been shown to have many antibodies present against it in early MS.[13] The other is myelin oligodendrocyte glycoproteins (MOG). Both "have been identified as targets of the immune response,[14]" Tying back into the “antibody-mediated demyelination” theory, these antibodies that result from the immune response might be factors that contribute to the development of multiple sclerosis.[14]
In one particular study,[13] the experiment calls for serums of anti-MOG and anti-MBP to test their tendencies on causing conversion of a "clinically isolated syndrome" in a patient to develop into "clinically definite" MS. There is the control group, who receives no serum, the group who receives both serums, and a group who receives only the anti-MOG serum. The results revealed that 23 percent of the patients who received no serum had a relapse after 45.1±13.7 months. 83 percent of the patients who had only the anti-MOG serum had a relapsed after 14.6±9.6 months. All but one of the 22 patients who had both serums relapsed, and happened in 7.5 ± 4.4 months.[13]
Given the results, a patient with a “clinically isolated syndrome” that appears headed for MS still has a highly varied prognosis and does not necessarily become “clinically definite” MS. The results are consistent with previous data on the disease. For instance, 30-40 percent of MS cases are said to be relatively benign, and in this study 38 percent of the patients were negative for the serums. This suggests that in the infancy of a disease, the “antibody status” can identify patients “who are likely to have a relatively benign[13]” case of the disease. The article also emphasizes that these results do not prove that these antibodies are causing the demyelination or apart of a larger process leading to demyelination. One practical application of this experiment and the significance of these results is that currently an MRI has to be used to assess a patient’s risk of developing the first relapse of MS. These results suggest that this cheaper and easier to perform procedure of measuring antibodies has the potential to achieve the same diagnosis.
In a similar study,[15] the risk conversion for patients diagnosed with clinically isolated syndrome (CIS) to develop clinically definite MS was studied. The anti-myelin antibodies were investigated as the possible predictor for this risk conversion. While 90 percent of CIS patients develop clinically definite multiple sclerosis within so many months to years, the results showed that patients who recorded negatively for antibodies generally have a more favorable prognosis in the delay of this development. Patients who tested positive for antibodies were able to “benefit from early treatment.[15]” Over a 12 month period, 30 patients tested positive for antibodies. 22 of those patients had developed CDMS. Of the patients who tested negative for antibodies, none of them developed CDMS.
In spite of these findings, another study suggests that these studies do not conclusively dictate that MOG is indeed one of the primary contributors in the pathogenic pathway for MS.[16] MOG has shown the ability to lead to “demyelination in vitro and in experimental animals”. And it has been found both in nerve-tissue lesions, as well as in patients diagnosed with multiple sclerosis. Still, the significance of these findings are not conclusive. Two other studies have only been able to confirm the results presented in the studies mentioned “in a subgroup analysis”. And “three other studies obtained negative results”. This particular study provides an alternative outcome to the given findings by suggesting that this anti-MOG antibody correlation to the development of MS “may at least in part reflect cross-reactivity between MOG and Epstein-Barr nuclear antigen.[16]”
[5] With MOG only being synthesized in the CNS, it has become associated with MS. But the true link between MOG and MS is still very controversial, particularly because of the lack of evidence supporting the link between “biologically active anti-MOG antibodies[5]” and the demyelination that leads to multiple sclerosis. And, while the anti-MOG antibodies are able to be measured in determining the extent of damage in the tissue caused by MS, “apart from biologically active antibodies[5]”, it may be that “antibodies are just a bystander phenomenon of CNS tissue destruction[5]”.
References
- ^ a b PDB 1PKO; Breithaupt C, Schubart A, Zander H, Skerra A, Huber R, Linington C, Jacob U (August 2003). "Structural insights into the antigenicity of myelin oligodendrocyte glycoprotein". Proc. Natl. Acad. Sci. U.S.A. 100 (16): 9446–51. doi:10.1073/pnas.1133443100. PMC 170938. PMID 12874380. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=170938.
- ^ Pham-Dinh D, Della Gaspera B, Kerlero de Rosbo N, Dautigny A (September 1995). "Structure of the human myelin/oligodendrocyte glycoprotein gene and multiple alternative spliced isoforms". Genomics 29 (2): 345–52. doi:10.1006/geno.1995.9995. PMID 8666381.
- ^ Pham-Dinh D, Jones EP, Pitiot G, Della Gaspera B, Daubas P, Mallet J, Le Paslier D, Fischer Lindahl K, Dautigny A (1995). "Physical mapping of the human and mouse MOG gene at the distal end of the MHC class Ib region". Immunogenetics 42 (5): 386–91. PMID 7590972.
- ^ a b c d e f g h Roth MP, Malfroy L, Offer C, Sevin J, Enault G, Borot N, Pontarotti P, Coppin H (July 1995). "The human myelin oligodendrocyte glycoprotein (MOG) gene: complete nucleotide sequence and structural characterization". Genomics 28 (2): 241–50. doi:10.1006/geno.1995.1137. PMID 8530032.
- ^ a b c d e f g h i j Berger, T., Innsbruck Medical University Dept. of Neurology interviewed by S. Gillooly, Nov. 24, 2008.
- ^ Pham-Dinh D, Allinquant B, Ruberg M, Della Gaspera B, Nussbaum JL, Dautigny A (December 1994). "Characterization and expression of the cDNA coding for the human myelin/oligodendrocyte glycoprotein". J. Neurochem. 63 (6): 2353–6. doi:10.1046/j.1471-4159.1994.63062353.x. PMID 7964757. http://www.blackwell-synergy.com/openurl?genre=article&sid=nlm:pubmed&issn=0022-3042&date=1994&volume=63&issue=6&spage=2353.
- ^ Pham-Dinh D, Mattei MG, Nussbaum JL, Roussel G, Pontarotti P, Roeckel N, Mather IH, Artzt K, Lindahl KF, Dautigny A (September 1993). "Myelin/oligodendrocyte glycoprotein is a member of a subset of the immunoglobulin superfamily encoded within the major histocompatibility complex". Proc. Natl. Acad. Sci. U.S.A. 90 (17): 7990–4. doi:10.1073/pnas.90.17.7990. PMC 47273. PMID 8367453. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=47273.
- ^ a b c Berger T, Reindl M (August 2007). "Multiple sclerosis: disease biomarkers as indicated by pathophysiology". J. Neurol. Sci. 259 (1-2): 21–6. doi:10.1016/j.jns.2006.05.070. PMID 17367811.
- ^ Boyle, L.H., Traherne, J.A., Plotnek, G et Splice variation in the cytoplasmic domains of myelin oligodendrocyte glycoprotein affects its cellular localisation and transport. Journal of Neurochemistry, 2007, 102, 1853-62.
- ^ Kabsch W, Sander C (December 1983). "Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features". Biopolymers 22 (12): 2577–637. doi:10.1002/bip.360221211. PMID 6667333.
- ^ Murzin AG, Brenner SE, Hubbard T, Chothia C (April 1995). "SCOP: a structural classification of proteins database for the investigation of sequences and structures". J. Mol. Biol. 247 (4): 536–40. doi:10.1016/S0022-2836(05)80134-2. PMID 7723011.
- ^ Clements CS, Reid HH, Beddoe T, et al. (September 2003). "The crystal structure of myelin oligodendrocyte glycoprotein, a key autoantigen in multiple sclerosis". Proc. Natl. Acad. Sci. U.S.A. 100 (19): 11059–64. doi:10.1073/pnas.1833158100. PMC 196926. PMID 12960396. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=196926.
- ^ a b c d e Berger T, Rubner P, Schautzer F, Egg R, Ulmer H, Mayringer I, Dilitz E, Deisenhammer F, Reindl M (July 2003). "Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event". N. Engl. J. Med. 349 (2): 139–45. doi:10.1056/NEJMoa022328. PMID 12853586.
- ^ a b c Tomassini V, De Giglio L, Reindl M, Russo P, Pestalozza I, Pantano P, Berger T, Pozzilli C (November 2007). "Anti-myelin antibodies predict the clinical outcome after a first episode suggestive of MS". Mult. Scler. 13 (9): 1086–94. doi:10.1177/1352458507077622. PMID 17468447.
- ^ a b Greeve I, Sellner J, Lauterburg T, Walker U, Rösler KM, Mattle HP (October 2007). "Anti-myelin antibodies in clinically isolated syndrome indicate the risk of multiple sclerosis in a Swiss cohort". Acta Neurol. Scand. 116 (4): 207–10. doi:10.1111/j.1600-0404.2007.00872.x. PMID 17824895.
- ^ a b Wang H, Munger KL, Reindl M, O'Reilly EJ, Levin LI, Berger T, Ascherio A (October 2008). "Myelin oligodendrocyte glycoprotein antibodies and multiple sclerosis in healthy young adults". Neurology 71 (15): 1142–6. doi:10.1212/01.wnl.0000316195.52001.e1. PMID 18753473.
External links
PDB gallery Arrestin Membrane-spanning 4A Myelin Myelin basic protein (PMP2) · Myelin proteolipid protein (PLP1) · Myelin oligodendrocyte glycoprotein · Myelin-associated glycoprotein · Myelin protein zeroPulmonary surfactant Tetraspanin TSPAN1 · TSPAN2 · TSPAN3 · TSPAN4 · TSPAN5 · TSPAN6 · TSPAN7 · TSPAN8 · TSPAN9 · TSPAN10 · TSPAN11 · TSPAN12 · TSPAN13 · TSPAN14 · TSPAN15 · TSPAN16 · TSPAN17 · TSPAN18 · TSPAN19 · TSPAN20 · TSPAN21 · TSPAN22 · TSPAN23 · TSPAN24 · TSPAN25 · TSPAN26 · TSPAN27 · TSPAN28 · TSPAN29 · TSPAN30 · TSPAN31 · TSPAN32 · TSPAN33 · TSPAN34Other/ungrouped Calnexin · LDL-receptor-related protein associated protein · Neurofibromin 2 · Presenilin (PSEN1, PSEN2) · HFE · Phospholipid transfer proteins · Dysferlin · STRC · OTOFsee also other cell membrane protein disorders
B memb: cead, trns (1A, 1C, 1F, 2A, 3A1, 3A2-3, 3D), othrCategories:- Human proteins
- Glycoproteins
Wikimedia Foundation. 2010.