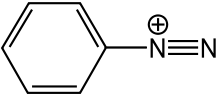
- Diazonium compound
-
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group R-N2+ X- where R can be any organic residue such alkyl or aryl and X is an inorganic or organic anion such as a halogen. Diazonium salts, especially those where R is an aryl group, are important intermediates in the organic synthesis of azo dyes.[1]
Contents
Preparation
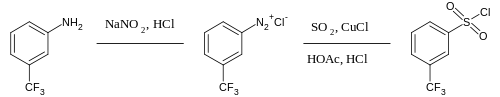
The process of forming diazonium compounds is called "diazotation", "diazoniation", or "diazotization". The reaction was first reported by Peter Griess in 1858, who subsequently discovered several reactions of this new class of compounds. The most important method for the preparation of diazonium salts is treatment of aromatic amines such as aniline with nitrous acid. Usually the nitrous acid is generated in situ (in the same flask) from sodium nitrite and mineral acid. In aqueous solution diazonium salts are unstable at temperatures above +5 °C; the -N+≡N group tends to be lost as N2 (nitrogen gas). One can isolate diazonium compounds as tetrafluoroborate salts, which are stable at room temperature. Often, diazonium compounds are not isolated and once prepared, used immediately in further reactions. This approach is illustrated in the preparation of an arylsulfonyl compound:[2]
It is often preferred that the diazonium salt remain in solutions, but they do tend to supersaturate. Operators have been killed and injured by an unexpected crystallization of the salt followed by its detonation.[3]
Reactions
Displacement of N2 group
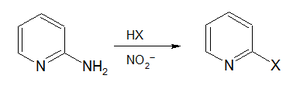
The diazo group (N2) can be displaced in a process called dediazoniation, which releases nitrogen N2 and an aryl carbocation or more commonly in combination with single electron transfer an aryl radical.[4] Dediazotization is commonly induced by halides. The process is a formal nucleophilic aromatic substitution reaction, is the basis of the Sandmeyer Reaction, the Gomberg-Bachmann reaction and the Schiemann reaction. In the so-called Craig method, 2-aminopyridine reacts with sodium nitrite, hydrobromic acid and excess bromine to 2-bromopyridine.[5]
Several other methods exist for dediazotization:
- by organic reduction at an electrode
- by gamma radiation from solvated electrons generated in water
- photoinduced electron transfer
- reduction by metal cations, most commonly a cuprous salt.
- anion-induced dediazoniation: a counterion such as iodine gives electron transfer to the diazonium cation forming the aryl radical and an iodine radical
- solvent-induced dediazoniation with solvent serving as electron donor
Diazo coupling
An important reaction of aromatic diazonium salts is azo coupling. In this process, the diazonium compound attacks electron-rich arenes such as anilines and phenols concomitant with release of a proton. The process is an example of electrophilic aromatic substitution:
- ArN2+ + Ar'H → ArN2Ar' + H+
The resulting azo compounds are often useful dyes and in fact are called azo dyes.[6] The deep colors of the dyes reflects their extended conjugation. For example, the dye called aniline yellow is produced by mixing aniline and cold solution of diazonium salt and then shaking it vigorously. Aniline yellow is obtained as an yellow solid.[7] Similarly, a cold basic solution of Naphthalen-2-ol (Β-naphthol) give the intensely orange-red precipitate.[7] Methyl orange is an example of an azo dye that is used in the laboratory as a pH indicator.
Other reactions
- In Meerwein arylation the salt also decomposes and the aryl residue reacts with an electron deficient alkene in an addition reaction
- In the Bamberger triazine synthesis and the Widman-Stoermer synthesis a diazonium salt reacts as an electrophile through its terminal nitrogen atom with an activated double bond.
- Hydrolysis of diazonium salts yields alcohols
- Reduction with hypophosphorous acid replaces the nitrogen by hydrogen, which allows amino (and, indirectly, nitro groups) to be removed
- In the Heck-Matsuda Reaction the salt couples with an alkene.
Metal complexes
Diazonium cations are similar to NO+ and thus form complexes with many metal centers, especially in organometallic chemistry. Such compounds are usually prepared by direct reaction of the low-valent metal complexes with diazonium salts. Illustrative complexes are [Fe(CO)2(PPh3)2(N2Ph)]+ and the chiral-at-metal complex Fe(CO)(NO)(PPh3)(N2Ph).[8]
Grafting reactions
In a potential application in nanotechnology, the diazonium salts 4-chlorobenzenediazonium tetrafluoroborate very efficiently functionalizes single wall nanotubes.[9] In order to exfoliate the nanotubes, they are mixed with an ionic liquid in a mortar and pestle. The diazonium salt is added together with potassium carbonate, and after grinding the mixture at room temperature the surface of the nanotubes are covered with chlorophenyl groups with an efficiency of 1 in 44 carbon atoms. These added subsituents prevent the tubes from forming intimate bundles due to large cohesive forces between them, which is a recurring problem in nanotube technology.
It is also possible to functionalize silicon wafers with diazonium salts forming an aryl monolayer. In one study, the silicon surface is washed with ammonium hydrogen fluoride leaving it covered with silicon-hydrogen bonds (hydride passivation).[10] The reaction of the surface with a solution of diazonium salt in acetonitrile for 2 hours in the dark is a spontaneous process through a free radical mechanism:[11]
So far grafting of diazonium salts on metals has been accomplished on iron, cobalt, nickel, platinum, palladium, zinc, copper and gold surfaces. Also the grafting to diamond surfaces has been reported.[12] One interesting question raised is the actual positioning on the aryl group on the surface. An in silico study [13] demonstrates that in the period 4 elements from titanium to copper the binding energy decreases from left to right because the number of d-electrons increases. The metals to the left of iron are positioned tilted towards or flat on the surface favoring metal to carbon pi bond formation and those on the right of iron are positioned in an upright position, favoring metal to carbon sigma bond formation. This also explains why diazonium salt grafting thus far has been possible with those metals to right of iron in the periodic table.
Applications
The first use of diazonium salts was to produce water-fast dyed fabrics by immersing the fabric in an aqueous solution of the diazonium compound, followed by immersion in a solution of the coupler (the electron-rich ring that undergoes electrophilic substitution). The major current use remains in the dye industry.[6]
Organic synthesis
As discussed above under reactions, diazonium compounds are useful in the preparation of substituted aromatic compounds from anilines. Fluorobenzene for example is prepared by the thermal decomposition of the phenyldiazonium tetrafluoroborate:[14]
Niche uses
Diazonium salts are light sensitive and break down under near UV or violet light. This property has led to their use in document reproduction. In this process, paper or film is coated with a diazonium salt. After contact exposure under light, the residual diazo is converted to a stable azo dye with an aqueous solution of coupler. A more common process uses a paper coated with diazo, coupler and an acid to inhibit coupling; after exposure the image is developed by a vapor mixture of ammonia and water which forces coupling.
Safety
Diazonium salts are often dangerously explosive, and fatalities and injuries have been reported. In fact, aryl diazonium perchlorates, such as nitrobenzenediazonium perchlorate which are relatively stable, have been used in initiating explosives.
See also
References
- ^ Chemistry of the Diazonium and Diazo Groups: Part 1. S. Patai, Ed. 1978 Wiley-Blackwell. ISBN 0471994928. Chemistry of the Diazonium and Diazo Groups: Part 2. S. Patai, Ed. 1978 Wiley-Blackwell. ISBN 0471994936.
- ^ R. V. Hoffman (1990), "m-Trifluoromethylbenzenesulfonyl Chloride", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV7P0508; Coll. Vol. 7: 508
- ^ "UK CRHF Incident Report - Supersaturated Diazonium salt causes Fatality". http://www.crhf.org.uk/incident71.html. Retrieved 13 May 2010.
- ^ Carlo Galli (1988). "Radical reactions of arenediazonium ions: An easy entry into the chemistry of the aryl radical". Chem. Rev. 88 (5): 765. doi:10.1021/cr00087a004.
- ^ Lyman C. Craig (1934). "A Study of the Preparation of Alpha-Pyridyl Halides from Alpha-Aminopyridine by the Diazo Reaction". J. Am. Chem. Soc. 56 (1): 231–232. doi:10.1021/ja01316a072.
- ^ a b Klaus Hunger, Peter Mischke, Wolfgang Rieper, Roderich Raue, Klaus Kunde, Aloys Engel "Azo Dyes” in Ullmann’s Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim.doi:10.1002/14356007.a03_245.
- ^ a b Clark, Jim. "chemguide". http://www.chemguide.co.uk/organicprops/aniline/propsdiazo.html. Retrieved 28 September 2011.
- ^ Sutton, D., "Organometallic Diazo Compounds", Chem. Rev., 1993, volume 93, 905-1022. doi:10.1021/cr00019a008
- ^ Green Chemical Functionalization of Single-Walled Carbon Nanotubes in Ionic Liquids B. Katherine Price, Jared L. Hudson, and James M. Tour J. Am. Chem. Soc.; 2005; 127(42) pp 14867 - 14870. doi:10.1021/ja053998c
- ^ Michael P. Stewart, Francisco Maya, Dmitry V. Kosynkin, Shawn M. Dirk, Joshua J. Stapleton, Christine L. McGuiness, David L. Allara, and James M. Tour (2004). "Direct Covalent Grafting of Conjugated Molecules onto Si, GaAs, and Pd Surfaces from Aryldiazonium Salts". J. Am. Chem. Soc. 126 (1): 370. doi:10.1021/ja0383120. PMID 14709104.
- ^ Reaction sequence: silicon surface reaction with ammonium hydrogen fluoride creates hydride layer. An electron is transferred from the silicon surface to the diazonium salt in an open circuit potential reduction leaving a silicon radical cation and a diazonium radical. In the next step a proton and a nitrogen molecule are expelled and the two radical residues recombine creating a surface silicon to carbon bond.
- ^ S.Q. Lud, M. Steenackers, P. Bruno, D.M. Gruen, P. Feulner, J.A. Garrido, and M. Stutzmann (2006). "Chemical Grafting of Biphenyl Self-Assembled Monolayers on Ultrananocrystalline Diamond". J. Am. Chem. Soc. 128 (51): 16884. doi:10.1021/ja0657049. PMID 17177439.
- ^ De-en Jiang, Bobby G. Sumpter, and Sheng Dai (2006). "Structure and Bonding between an Aryl Group and Metal Surfaces". J. Am. Chem. Soc. 128 (18): 6030. doi:10.1021/ja061439f. PMID 16669660.
- ^ Flood, D. T. (1943), "Fluorobenzene", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV2P0295; Coll. Vol. 2: 295.
External links
- W. Reusch. "Reactions of Amines". VirtualText of Organic Chemistry. Michigan State University. http://www.cem.msu.edu/~reusch/VirtualText/amine2.htm.
Categories:- Organic compounds
- Carbon-heteroatom bond forming reactions
Wikimedia Foundation. 2010.