- Dolutegravir
-
Dolutegravir 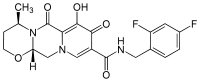
Identifiers CAS number 1051375-16-6 ChemSpider 25991412 Jmol-3D images Image 1 - C[C@@H]1CCO[C@@H]2N1Cc3c(c(=O)c(cn3C2)C(=O)NCc4ccc(cc4F)F)O
- InChI=InChI=1S/C20H21F2N3O4/c1-11-4-5-29-17-10-24-8-14(18(26)19(27)16(24)9-25(11)17)20(28)23-7-12-2-3-13(21)6-15(12)22/h2-3,6,8,11,17,27H,4-5,7,9-10H2,1H3,(H,23,28)/t11-,17+/m1/s1
Properties Molecular formula C20H21F2N3O4 Molar mass 405.4 g mol−1 Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references Dolutegravir is an experimental new drug under investigation for the treatment of HIV infection. Dolutegravir is an integrase inhibitor. Also known as S/GSK1349572 or just "572", the drug is under development by GlaxoSmithKline (GSK). Studies have shown dolutegravir to be effective in patients with resistance to the only currently available integrase inhibitor, raltegravir.[1] Clinical trials are underway to support dolutegravir in combination with abacavir and lamivudine, in a new new fixed dose combination called 572-Trii.[2]
References
- ^ Dolutegravir ("572") Holds Up in Heavily Raltegravir-Resistant Patients, Phase 2B Study Finds Nelson Vergel. The Body PRO. Accessed 23 April 2011.
- ^ Shionogi-ViiV Healthcare Starts Phase 3 Trial for "572-Trii" Test positive airwave. The Body PRO. Accessed 23 April 2011.

This antiinfective drug article is a stub. You can help Wikipedia by expanding it.
