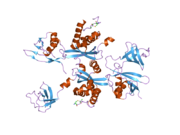- Neutrophil cytosolic factor 4
-
Neutrophil cytosolic factor 4, 40kDa 
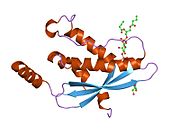
PDB rendering based on 1h6h.Available structures PDB 1H6H, 1OEY, 1W6X, 1W70, 1Z9Q, 2DYB Identifiers Symbols NCF4; MGC3810; NCF; P40PHOX; SH3PXD4 External IDs OMIM: 601488 MGI: 109186 HomoloGene: 525 GeneCards: NCF4 Gene Gene Ontology Molecular function • protein binding
• phosphatidylinositol binding
• protein dimerization activityCellular component • cytoplasm
• cytosol
• membrane
• NADPH oxidase complexBiological process • immune response
• cell communication
• oxidation-reduction processSources: Amigo / QuickGO RNA expression pattern 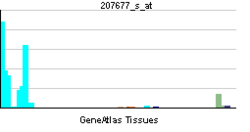
More reference expression data Orthologs Species Human Mouse Entrez 4689 17972 Ensembl ENSG00000100365 ENSMUSG00000071715 UniProt Q15080 Q3TBC6 RefSeq (mRNA) NM_000631.4 NM_008677.2 RefSeq (protein) NP_000622.2 NP_032703.2 Location (UCSC) Chr 22:
37.26 – 37.27 MbChr 15:
78.08 – 78.09 MbPubMed search [1] [2] Neutrophil cytosol factor 4 is a protein that in humans is encoded by the NCF4 gene.[1][2]
The protein encoded by this gene is a cytosolic regulatory component of the superoxide-producing phagocyte NADPH-oxidase, a multicomponent enzyme system important for host defense. This protein is preferentially expressed in cells of myeloid lineage. It interacts primarily with neutrophil cytosolic factor 2 (NCF2/p67-phox) to form a complex with neutrophil cytosolic factor 1 (NCF1/p47-phox), which further interacts with the small G protein RAC1 and translocates to the membrane upon cell stimulation. This complex then activates flavocytochrome b, the membrane-integrated catalytic core of the enzyme system. The PX domain of this protein can bind phospholipid products of the PI(3) kinase, which suggests its role in PI(3) kinase-mediated signaling events. The phosphorylation of this protein was found to negatively regulate the enzyme activity. Alternatively spliced transcript variants encoding distinct isoforms have been observed.[2]
Interactions
Neutrophil cytosolic factor 4 has been shown to interact with Ku70,[3] Neutrophil cytosolic factor 1[4][5][6] and Moesin.[7]
References
- ^ Zhan S, Vazquez N, Zhan S, Wientjes FB, Budarf ML, Schrock E, Ried T, Green ED, Chanock SJ (Nov 1996). "Genomic structure, chromosomal localization, start of transcription, and tissue expression of the human p40-phox, a new component of the nicotinamide adenine dinucleotide phosphate-oxidase complex". Blood 88 (7): 2714–21. PMID 8839867.
- ^ a b "Entrez Gene: NCF4 neutrophil cytosolic factor 4, 40kDa". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=4689.
- ^ Grandvaux, N; Grizot S, Vignais P V, Dagher M C (Feb. 1999). "The Ku70 autoantigen interacts with p40phox in B lymphocytes". J. Cell. Sci. (ENGLAND) 112 ( Pt 4): 503–13. ISSN 0021-9533. PMID 9914162.
- ^ Lapouge, Karine; Smith Susan J M, Groemping Yvonne, Rittinger Katrin (Mar. 2002). "Architecture of the p40-p47-p67phox complex in the resting state of the NADPH oxidase. A central role for p67phox". J. Biol. Chem. (United States) 277 (12): 10121–8. doi:10.1074/jbc.M112065200. ISSN 0021-9258. PMID 11796733.
- ^ Grizot, S; Grandvaux N, Fieschi F, Fauré J, Massenet C, Andrieu J P, Fuchs A, Vignais P V, Timmins P A, Dagher M C, Pebay-Peyroula E (Mar. 2001). "Small angle neutron scattering and gel filtration analyses of neutrophil NADPH oxidase cytosolic factors highlight the role of the C-terminal end of p47phox in the association with p40phox". Biochemistry (United States) 40 (10): 3127–33. doi:10.1021/bi0028439. ISSN 0006-2960. PMID 11258927.
- ^ Sathyamoorthy, M; de Mendez I, Adams A G, Leto T L (Apr. 1997). "p40(phox) down-regulates NADPH oxidase activity through interactions with its SH3 domain". J. Biol. Chem. (UNITED STATES) 272 (14): 9141–6. doi:10.1074/jbc.272.14.9141. ISSN 0021-9258. PMID 9083043.
- ^ Wientjes, F B; Reeves E P, Soskic V, Furthmayr H, Segal A W (Nov. 2001). "The NADPH oxidase components p47(phox) and p40(phox) bind to moesin through their PX domain". Biochem. Biophys. Res. Commun. (United States) 289 (2): 382–8. doi:10.1006/bbrc.2001.5982. ISSN 0006-291X. PMID 11716484.
Further reading
- Matute JD, Arias AA, Dinauer MC, Patiño PJ (2006). "p40phox: the last NADPH oxidase subunit.". Blood Cells Mol. Dis. 35 (2): 291–302. doi:10.1016/j.bcmd.2005.06.010. PMID 16102984.
- Jones JH (1977). "The essence of operating room nursing. Paramedical personnel.". The Australasian nurses journal 7 (1): 44–5, 63–4. PMID 243433.
- Leto TL, Adams AG, de Mendez I (1994). "Assembly of the phagocyte NADPH oxidase: binding of Src homology 3 domains to proline-rich targets.". Proc. Natl. Acad. Sci. U.S.A. 91 (22): 10650–4. doi:10.1073/pnas.91.22.10650. PMC 45079. PMID 7938008. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=45079.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides.". Gene 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Tsunawaki S, Mizunari H, Nagata M, et al. (1994). "A novel cytosolic component, p40phox, of respiratory burst oxidase associates with p67phox and is absent in patients with chronic granulomatous disease who lack p67phox.". Biochem. Biophys. Res. Commun. 199 (3): 1378–87. doi:10.1006/bbrc.1994.1383. PMID 8147882.
- Wientjes FB, Hsuan JJ, Totty NF, Segal AW (1994). "p40phox, a third cytosolic component of the activation complex of the NADPH oxidase to contain src homology 3 domains.". Biochem. J. 296 ( Pt 3): 557–61. PMC 1137734. PMID 8280052. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1137734.
- Dusi S, Donini M, Rossi F (1996). "Mechanisms of NADPH oxidase activation: translocation of p40phox, Rac1 and Rac2 from the cytosol to the membranes in human neutrophils lacking p47phox or p67phox.". Biochem. J. 314 ( Pt 2): 409–12. PMC 1217064. PMID 8670049. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1217064.
- Someya A, Nagaoka I, Nunoi H, Yamashita T (1997). "Translocation of guinea pig p40-phox during activation of NADPH oxidase.". Biochim. Biophys. Acta 1277 (3): 217–25. PMID 8982388.
- Sathyamoorthy M, de Mendez I, Adams AG, Leto TL (1997). "p40(phox) down-regulates NADPH oxidase activity through interactions with its SH3 domain.". J. Biol. Chem. 272 (14): 9141–6. doi:10.1074/jbc.272.14.9141. PMID 9083043.
- Grogan A, Reeves E, Keep N, et al. (1998). "Cytosolic phox proteins interact with and regulate the assembly of coronin in neutrophils.". J. Cell. Sci. 110 ( Pt 24): 3071–81. PMID 9365277.
- Fuchs A, Bouin AP, Rabilloud T, Vignais PV (1997). "The 40-kDa component of the phagocyte NADPH oxidase (p40phox) is phosphorylated during activation in differentiated HL60 cells.". Eur. J. Biochem. 249 (2): 531–9. doi:10.1111/j.1432-1033.1997.00531.x. PMID 9370364.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, et al. (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library.". Gene 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Bouin AP, Grandvaux N, Vignais PV, Fuchs A (1998). "p40(phox) is phosphorylated on threonine 154 and serine 315 during activation of the phagocyte NADPH oxidase. Implication of a protein kinase c-type kinase in the phosphorylation process.". J. Biol. Chem. 273 (46): 30097–103. doi:10.1074/jbc.273.46.30097. PMID 9804763.
- Grandvaux N, Grizot S, Vignais PV, Dagher MC (1999). "The Ku70 autoantigen interacts with p40phox in B lymphocytes.". J. Cell. Sci. 112 ( Pt 4): 503–13. PMID 9914162.
- Nishiyama A, Ohno T, Iwata S, et al. (1999). "Demonstration of the interaction of thioredoxin with p40phox, a phagocyte oxidase component, using a yeast two-hybrid system.". Immunol. Lett. 68 (1): 155–9. doi:10.1016/S0165-2478(99)00045-0. PMID 10397171.
- Hasebe T, Someya A, Nagaoka I (1999). "Identification of a splice variant mRNA of p40phox, an NADPH oxidase component of phagocytes.". FEBS Lett. 455 (3): 257–61. doi:10.1016/S0014-5793(99)00905-9. PMID 10437784.
- Dunham I, Shimizu N, Roe BA, et al. (1999). "The DNA sequence of human chromosome 22.". Nature 402 (6761): 489–95. doi:10.1038/990031. PMID 10591208.
- Vergnaud S, Paclet MH, El Benna J, et al. (2000). "Complementation of NADPH oxidase in p67-phox-deficient CGD patients p67-phox/p40-phox interaction.". Eur. J. Biochem. 267 (4): 1059–67. doi:10.1046/j.1432-1327.2000.01097.x. PMID 10672014.
- Grizot S, Grandvaux N, Fieschi F, et al. (2001). "Small angle neutron scattering and gel filtration analyses of neutrophil NADPH oxidase cytosolic factors highlight the role of the C-terminal end of p47phox in the association with p40phox.". Biochemistry 40 (10): 3127–33. doi:10.1021/bi0028439. PMID 11258927.
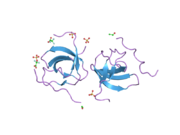
PDB gallery 1h6h: STRUCTURE OF THE PX DOMAIN FROM P40PHOX BOUND TO PHOSPHATIDYLINOSITOL 3-PHOSPHATE1oey: HETERODIMER OF P40PHOX AND P67PHOX PB1 DOMAINS FROM HUMAN NADPH OXIDASE1w6x: SH3 DOMAIN OF P40PHOX, COMPONENT OF THE NADPH OXIDASE1w70: SH3 DOMAIN OF P40PHOX COMPLEXED WITH C-TERMINAL POLYPROLINE REGION OF P47PHOX1z9q: Solution structure of SH3 domain of p40phox2dyb: The crystal structure of human p40(phox)Oxidoreductases: NADH or NADPH (EC 1.6) 1.6.1: NAD/NADP 1.6.2: Heme 1.6.3: Oxygen NADPH oxidase (P91-PHOX, Neutrophil cytosolic factor 1, Neutrophil cytosolic factor 2, Neutrophil cytosolic factor 4) · dual oxidase (Dual oxidase 1, Dual oxidase 2)1.6.5: Quinone or similar 1.6.6: Nitrogenous group 1.6.99: other Categories:- Human proteins
- Chromosome 22 gene stubs
Wikimedia Foundation. 2010.