- Primary cell
-
A primary cell is any kind of battery in which the electrochemical reaction is not reversible, rendering the cell non-rechargeable. A common example of a primary cell is the disposable battery. Unlike a secondary cell, the reaction cannot be reversed by running a current into the cell; the chemical reactants cannot be restored to their initial position and capacity. Primary batteries use up the materials in one or both of their electrodes.
Contents
Comparison with rechargeables
Rechargeable batteries are economical to use when their initially higher cost and cost of a charging system can be spread out over many use cycles; for example, in hand-held power tools, it would be very costly to replace a high-capacity primary battery pack every few hours of use.
Primary batteries are useful where long periods of storage are required; a primary battery can be constructed to have a lower self-discharge rate than a rechargeable battery, so all its capacity is available for useful purposes. Applications that require a small current for a long time, for example a smoke detector, also use primary batteries since the self-discharge current of a rechargeable battery would exceed the load current and limit service time to a few days or weeks. For example, a flashlight used for emergencies must work when needed, even if it has sat on a shelf for an extended period of time. Primary cells are also more cost-efficient in this case, as rechargeable batteries would use only a small fraction of available recharge cycles. Reserve batteries achieve very long storage time (on the order of 10 years or more) without loss of capacity, by physically separating the components of the battery and only assembling them at the time of use. Such constructions are expensive but are found in applications like munitions, which may be stored for years before use.
Polarization
A primary cell becomes polarized when in use. This means that hydrogen accumulates at the cathode and reduces the effectiveness of the cell. To remove the hydrogen, a depolarizer is used and this may be mechanical, chemical or electrochemical.
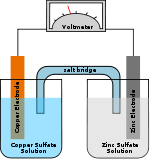
Attempts have been made to make simple cells self-depolarizing by roughening the surface of the copper plate to facilitate the detachment of hydrogen bubbles. These attempts have had little success. Chemical depolarization utilizes an oxidizing agent, such as manganese dioxide (e.g., Leclanché cell and zinc–carbon cell) or nitric acid (e.g., Bunsen cell and Grove cell), to oxidize the hydrogen to water. Electrochemical depolarization exchanges the hydrogen for a metal, such as copper (e.g., Daniell cell), or silver (e.g., Silver-oxide cell).
Terminology
Anode and cathode
The plate that carries the positive terminal (usually carbon) is termed the cathode and the plate that carries the negative terminal (usually zinc) is termed the anode. This is the reverse of the terminology used in an electrolytic cell. The reason is that the terms are related to the passage of electric current through the electrolyte, not the external circuit
Inside the cell the anode is the electrode where chemical oxidation occurs, as it accepts electrons from the electrolyte. The cathode is defined as the electrode where chemical reduction occurs, as it donates electrons to the electrolyte.
Outside the cell, different terminology is used. Since the anode accepts electrons from the electrolyte, it becomes negatively charged and is therefore connected to the terminal marked "−" on the outside of the cell. The cathode, meanwhile, donates electrons to the electrolyte, so it becomes positively charged and is therefore connected to the terminal marked "+" on the outside of the cell.[1]
Old textbooks sometimes contain different terminology that can cause confusion to modern readers. For example, a 1911 textbook by Ayrton and Mather[2] describes the electrodes as the "positive plate" and "negative plate" in a way that contradicts modern usage.
See also
- History of the battery
- Secondary cell (rechargeable)
- Fuel cell
- Battery recycling
- List of battery types
- Battery nomenclature
References
- ^ John S. Newman, Karen E. Thomas-Alyea, Electrochemical systems, Wiley-IEEE, 3rd ed. 2004, ISBN 0471477567
- ^ W. E. Ayrton and T. Mather, Practical Electricity, Cassell and Company, London, 1911, page 170
External links
Categories:
Wikimedia Foundation. 2010.