- Arsenic trisulfide
-
Arsenic trisulfide 
 Arsenic trisulfideOther namesArsenic(III) sulfide
Arsenic trisulfideOther namesArsenic(III) sulfide
OrpimentIdentifiers CAS number 1303-33-9 
PubChem 4093503 ChemSpider 21241348 
UNII 44SIJ800OX 
EC number 215-117-4 RTECS number CG2638000 Jmol-3D images Image 1 - S1[As]3S[As]2S[As](S[As]1S2)S3
Properties Molecular formula As2S3 Molar mass 246.04 g mol−1 Exact mass 245.759404904 g mol-1 Appearance Orange crystals Density 3.43 g cm-3 Melting point 310 °C, 583 K, 590 °F
Boiling point 707 °C, 980 K, 1305 °F
Structure Space group P21/n (No. 11) Lattice constant a = 1147.5(5) pm, b = 957.7(4) pm, c = 425.6(2) pm Lattice constant α = 90°, β = 90.68(8)°, γ = 90° pyramidal (As) Hazards[1][2] GHS pictograms 

GHS signal word DANGER GHS hazard statements H300, H331, H400, H411 EU Index 033-002-00-5 NFPA 704 U.S. Permissible
exposure limit (PEL)0.01 mg m−3 TWA (as As) Related compounds Other anions Arsenic trioxide
Arsenic triselenideOther cations Phosphorus trisulfide
Antimony trisulfide
Bismuth sulfideRelated compounds Tetraarsenic tetrasulfide  trisulfide (verify) (what is:
trisulfide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Arsenic trisulfide is the inorganic compound with the formula As2S3. This bright yellow solid is a well known mineral orpiment (Latin: auripigment), has been used as a pigment, and has played a role in the analysis of arsenic compounds. This chalcogenide material is a group V/VI, intrinsic p-type semiconductor and exhibits photo-induced phase-change properties. The other principal arsenic sulfide is realgar, As4S4, which is red-orange and also occurs as a mineral.
Contents
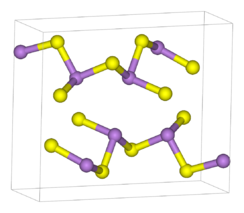
Structure
As2S3 occurs both in crystalline and amorphous forms. Both forms feature polymeric structures consisting trigonal pyramidal As(III) centres linked by sulfide centres. The sulfide centres are two-fold coordinated to two arsenic atoms. In the crystalline form, the compound adopts a ruffled sheet structure.[3] The bonding between the sheets consists of van der Waals forces. The crystalline form is usually found in geological samples. Amorphous As2S3 does not possess a layered structure but is more highly cross-linked. Like other glasses, there is no medium or long-range order, but the first co-ordination sphere is well defined. As2S3 is a good glass former and exhibits a wide glass-forming region in its phase diagram.
Synthesis and reactions
From the elements
Amorphous As2S3 is obtained via the fusion of the elements at 390 °C. Rapid cooling of the reaction melt ensures the disordered arrangement of the bonds, resulting in the glass. The reaction can be represented with the chemical equation:
- 2 As + 3 S → As2S3
Upon heating in a vacuum, polymeric As2S3 "cracks" to give a mixture of molecular species, including molecular As4S6.[4][5] As4S6 adopts the adamantane geometry, like that observed for P4O6 and As4O6. When a film of this material is exposed to an external energy source such as thermal energy (via thermal annealing [6]), electromagnetic radiation (i.e. UV lamps, lasers,[7] electron beams)[8]), As4S6 polymerizes:
Aqueous precipitation
See also: Qualitative inorganic analysisAs2S3 forms when aqueous solutions containing As(III) are treated with H2S. Arsenic was in the past analyzed and assayed by this reaction, which results in the precipitation of As2S3, which is then weighed. As2S3 can even be precipitated in 6M HCl. As2S3 is so insoluble that it is not toxic. As2S3 characteristically dissolves upon treatment with aqueous solutions containing sulfide ions. The dissolved arsenic species is the pyramidal trianion AsS3−
3:- As2S3 + 6 NaSH → 2 AsS3−
3 + 3 H2S
As2S3 is the anhydride of the hypothetical thioarsenous acid, As(SH)3. Upon treatment with polysulfide ions, As2S3 dissolves to give a variety of species containing both S-S and As-S bonds. One derivative is S7As-S-, a ring that contains an exocyclic sulfido center attached to the As atom. As2S3 also dissolves in strongly alkaline solutions to give a mixture of AsS3−
3 and AsO3−
3.[9]Reactions with oxygen
"Roasting" As2S3 in air gives volatile, toxic derivatives, this conversion being one of the hazards associated with the refining of heavy metal ores:
- 2 As2S3 + 9 O2 → As4O6 + 6 SO2
Contemporary uses
As an inorganic photoresist
Due to its high refractive index of 2.45 and its large Knoop hardness compared to organic photoresists, As2S3 has been investigated for the fabrication of photonic crystals with a full-photonic band-gap. Advances in laser patterning techniques such as three-dimensional direct laser writing (3-D DLW) and chemical wet-etching chemistry, has allowed this material to be used as a photoresist to fabricate 3-D nanostructures.[10][11]
As2S3 has been investigated for use as a high resolution photoreist material since the early 1970s,[12][13] using aqueous etchants. Although these aqueous etchants allowed for low-aspect ratio 2-D structures to be fabricated, they do not allow for the etching of high aspect ratio structures with 3-D periodicity. Certain organic reagents, used in organic solvents, permit the high-etch selectivity required to produce high-aspect ratio structures with 3-D periodicity.
Medical applications
As2S3 and As4S4 have been investigated as treatments for acute promyelocytic leukemia (APL).[14] The mode of action is thought to be similar to that for As2O3.
For IR-transmitting glasses
Arsenic trisulfide manufactured into amorphous form is used as a chalcogenide glass for infrared optics. It is transparent between 620 nm and 11 µm. The arsenic trisulfide glass is more resistant to oxidation than crystalline arsenic trisulfide, which minimizes toxicity concerns.[15] It can be also used as an acousto-optic material.
Role in ancient artistry
Main article: orpimentThe ancient Egyptians reportedly used orpiment, natural or synthetic, as a pigment in artistry and cosmetics.
Miscellaneous
Arsenic trisulfide is also used as a tanning agent. It was formerly used with indigo dye for the production of pencil blue, which allowed dark blue hues to be added to fabric via pencil or brush.
Precipitation of arsenic trisulfide is used as an analytical test for presence of dissimilatory arsenic-reducing bacteria (DARB).[16]
Safety
As2S3 is so insoluble that its toxicity is low. Aged samples can contain substantial amounts of arsenic oxides, which are soluble and therefore highly toxic.
Natural occurrence
Orpiment is found in volcanic environments, often together with other arsenic sulfides, mainly realgar. It is sometimes found in low-temperature hydrothermal veins, together with some other sulfide and sulfosalt minerals.
References
- ^ Index no. 033-002-00-5 of Annex VI, Part 3, to Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJEU L353, 31.12.2008, pp 1–1355 at p 427.
- ^ "Inorganic arsenic", 29 C.F.R. § 1910.1018, 58 FR 35310, June 30, 1993, as amended. "Arsenic (inorganic compounds, as As)", Pocket Guide to Chemical Hazards, U.S. Department of Health and Human Services (NIOSH) Publication No. 2005-149, Washington, DC: Government Printing Office, 2005, ISBN 9780160727511, http://www.cdc.gov/niosh/npg/npgd0038.html.
- ^ Wells, A.F. (1984). Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ^ Martin, T. P. Solid State Commun. 1983, 47, 2, pp 111.
- ^ Hammam, M. Santiago, J.J. Solid State Commun. 1986, 59, 11, 725.
- ^ Street, R.A., Nemanich, R.J., Connell, G.A.N. Phys. Rev. B, 1978, 18, 12, pp 6915.
- ^ Zoubir, A.; Richardson, M.; Rivero, C.; Schulte, A.; Lopez, C.; Richardson, K. Opt. Lett. 2004, 29, 7, 748.
- ^ Nordman, O., Nordman, N., Peyghambarian, N. J. Appl. Phys. 1998, 84, 11, pp 6055.
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Wong, S.; Deubel, M.; Pérez-Willard, F.; John, S.; Ozin, G. A.; Wegener, M.; von Freymann, G. Adv. Mater. 2006, 18, pp 265 - 269.
- ^ Wong S.; Thiel, M.; Brodersen, P.; Fenske, D.; Ozin, G. A.; Wegener, M.; von Freymann, G. Chem. Mater. 2007, volume 19, pp 4213-4221.
- ^ Stoycheva, R; Simidchieva, P.; Buroff, A. J. Non-Cryst. Solids 1987, volume 90, pp 541.
- ^ Zenkin, S. A.; Mamedov, S. B.; Mikhailov, M. D.; Turkina, E. Yu.; Yusupov, I. Yu. Glass Phys. Chem. 1997, 5, pp 393-399.
- ^ D.-P. Lu, J.-Y. Qiu, B. Jiang, Q. Wang, K.-Y. Liu, Y.-R. Liu, S.-S. Chen "Tetra-arsenic tetra-sulfide for the treatment of acute promyelocytic leukemia: a pilot report" Blood 2002, Volume 99, pp. 3136-3143.
- ^ Material Safety Data Sheet
- ^ Linping Kuai, Arjun A. Nair, and Martin F. Polz "Rapid and Simple Method for the Most-Probable-Number Estimation of Arsenic-Reducing Bacteria" Appl Environ Microbiol. 2001, vol. 67, 3168–3173. DOI: 10.1128/AEM.67.7.3168-3173.2001.
Further reading
- "Arsenic and arsenic compounds", Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Supplement 7, Lyon, France: International Agency for Research on Cancer, 1987, pp. 100–6, ISBN 92-832-1411-0, http://monographs.iarc.fr/ENG/Monographs/suppl7/Suppl7-19.pdf. "Arsenic in Drinking Water", Some Drinking-water Disinfectants and Contaminants, including Arsenic, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 84, Lyon, France: International Agency for Research on Cancer, 2004, pp. 39–267, ISBN 92-832-1284-3, http://monographs.iarc.fr/ENG/Monographs/vol84/mono84-6.pdf.
- "Arsenic Compounds, Inorganic", Report on Carcinogens, Eleventh Edition, U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program, 2005, http://ntp.niehs.nih.gov/ntp/roc/eleventh/profiles/s015arse.pdf.
External links
- UK Poison Information Document
- IPCS Environmental Health Criteria 182: Arsenic
- IPCS Poisons Information Monograph G042: Arsenic
- WHO Food Additives Series 24
- JECFA Evaluation: Arsenic
- Arsenic trisulfide
Arsenic compounds Categories:- Chalcoarsenics
- Sulfides
- Optical materials
- Non-oxide glasses
Wikimedia Foundation. 2010.

