- Orpiment
-
Orpiment 
OrpimentGeneral Category Sulfide mineral Chemical formula As2S3 Strunz classification 02.FA.30 Crystal symmetry Monoclinic 2/m Unit cell a = 11.475(5) Å, b = 9.577(4) Å, c = 4.256(2) Å, β = 90.45(5)°; Z=4 Identification Color Lemon-yellow to golden or brownish yellow Crystal habit Commonly in foliated columnar or fibrous aggregates; may be reniform or botryoidal; also granular or powdery; rarely as prismatic crystals Crystal system Monoclinic Prismatic Twinning On {100} Cleavage Perfect on {010}, imperfect on {100}; Tenacity Sectile Mohs scale hardness 1.5 - 2 Luster Resinous, pearly on cleavage surface Streak Pale lemon-yellow Diaphaneity Transparent Specific gravity 3.49 Optical properties Biaxial (-) Refractive index nα = 2.400 nβ = 2.810 nγ = 3.020 Birefringence δ = 0.620 Pleochroism In reflected light, strong, white to pale gray with reddish tint; in transmitted light, Y = yellow, Z = greenish yellow 2V angle Measured: 30° to 76°, Calculated: 62° Dispersion r > v, strong References [1][2][3] Orpiment, As2S3, is a common monoclinic arsenic sulfide mineral. It has a Mohs hardness of 1.5 to 2 and a specific gravity of 3.46. It melts at 300 °C to 325 °C. Optically it is biaxial (−) with refractive indices of a=2.4, b=2.81, g=3.02.
Orpiment is an orange to yellow mineral that is found worldwide, and occurs as a sublimation product in volcanic fumaroles, low temperature hydrothermal veins, hot springs and as a byproduct of the decay of another arsenic mineral, realgar. It is often found in association with realgar. It takes its name from the Latin auripigmentum (aurum − gold + pigmentum − pigment) because of its deep yellow color.
Contents
Historical uses
Orpiment was an important item of trade in the Roman Empire and was used as a medicine in China although it is highly toxic. It was also used as a fly poison and to poison arrows. Because of its striking color, it was also a favourite with alchemists searching for a way to make gold, both in China and the West.
Orpiment was ground, processed and used for centuries as a pigment in painting and for sealing wax, being one of the few clear, bright yellow pigments available to artists up until the 19th century. Orpiment presented problems, however, such as its extreme toxicity and its incompatibility with other common pigments like lead and copper-based substances such as verdigris and azurite. The use of orpiment as a pigment material ended almost entirely with the advent of the cadmium yellows and the various dye-based colors of the 19th century.
Contemporary uses
It is presently used in the production of infrared-transmitting glass, oil cloth, linoleum; in semiconductors and photoconductors, as a pigment and in fireworks. Mixed with two parts of slaked lime, orpiment is still very commonly used in rural India as a depilatory. It is also used in the tanning industry to remove hair from hides.
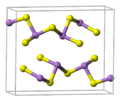
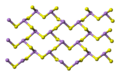
Crystal structure
-
orpiment's unit cell -
orpiment's crystal structure consists of sheets
References
- ^ http://rruff.geo.arizona.edu/doclib/hom/orpiment.pdf Handbook of Mineralogy
- ^ http://www.mindat.org/min-3021.html Mindat.org
- ^ http://webmineral.com/data/Orpiment.shtml Webmineral data
- The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. 11th Edition. Ed. Susan Budavari. Merck & Co., Inc., N.J., U.S.A. 1989.
- William Mesny. Mesny’s Chinese Miscellany. A Text Book of Notes on China and the Chinese. Shanghai. Vol. III, (1899), p. 251; Vol. IV, (1905), pp.26.
External links
- Webexhibits "Pigments Through the Ages: Orpiment"
- Babylonian Talmud Tractate Chullin see Rashi 'haZarnich' (Hebrew)
Categories:- Arsenic minerals
- Sulfide minerals
- Inorganic pigments
- Alchemical substances
- Poisonous minerals
- Monoclinic minerals
-
Wikimedia Foundation. 2010.