- Arsenic triiodide
-
Arsenic triiodide 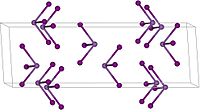 Arsenic triiodideSystematic nameTriiodoarsaneOther names
Arsenic triiodideSystematic nameTriiodoarsaneOther namesIdentifiers CAS number 7784-45-4 
PubChem 24575 
ChemSpider 22979 
EC number 232-068-4 RTECS number CG1950000 Jmol-3D images Image 1 - I[As](I)I
Properties Molecular formula AsI3 Molar mass 455.635 g/mol Appearance orange-red crystalline solid Density 4.69 g/cm3 Melting point 146 °C
Boiling point 403 °C
Solubility in water 6 g/100 mL Solubility soluble in alcohol, ether, CS2 Refractive index (nD) 2.23 Structure Crystal structure Rhombohedral, hR24, SpaceGroup = R-3, No. 148  triiodide (verify) (what is:
triiodide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Arsenic triiodide is the inorganic compound with the formula AsI3. It is a dark red solid that readily sublimes. It is a pyramidial molecule that is useful for preparing organoarsenic compounds.
Contents
Preparation
It is prepared by a reaction of arsenic trichloride and potassium iodide:[2]
- AsCl3 + 3KI → AsI3 + 3 KCl
Reactions
Hydrolysis occurs only slowly in water forming arsenic trioxide and hydroiodic acid. The reaction proceeds via formation of arsenous acid which exists in equilibrium with hydroiodic acid. The aqueous solution is highly acidic, pH of 0.1N solution is 1.1. It decomposes to arsenic trioxide, elemental arsenic and iodine when heated in air at 200 °C. The decomposition, however, commences at 100 °C and occurs with the liberation of iodine.
Former uses
Under the name of Donovan's solution, it was once recommended to treat rheumatism, arthritis, malaria, trypanosome infections, tuberculosis, and diabetes.[1]
References
- ^ a b Shakhashiri BZ, "Chemical of the Week: Arsenic", University of Wisconsin–Madison Chemistry Dept.
- ^ John C. Bailar, Jr. "Arsenic Triiodide" Inorganic Syntheses 1939, volume 1, pp. 103–104, 2007. doi:10.1002/9780470132326.ch36
Arsenic compounds Categories:- Halomonoarsanes
- Inorganic compound stubs
- Iodides
Wikimedia Foundation. 2010.
