- Arsenic pentafluoride
-
Arsenic pentafluoride 
 Arsenic pentafluorideOther namesArsenic(V) fluoride,
Arsenic pentafluorideOther namesArsenic(V) fluoride,
Arsorane, pentafluoro-Identifiers CAS number 7784-36-3 
PubChem 82223 ChemSpider 74203 
ChEBI CHEBI:30530 
Jmol-3D images Image 1 - F[As](F)(F)(F)F
Properties Molecular formula AsF5 Molar mass 169.9136 g mol-1 Appearance colorless gas Density 2.138 g cm-3[1] Melting point -79.8 ˚C[1]
Boiling point -52.8 ˚C[1]
Solubility in benzene soluble Hazards EU classification Toxic (T)
Dangerous for the environment (N)R-phrases R23/25, R50/53 S-phrases (S1/2), S20/21, S28, S45, S60, S61 NFPA 704 Related compounds Related group 5 fluorides Phosphorus pentafluoride
Antimony pentafluoride
Bismuth pentafluorideRelated compounds Arsenic pentachloride
Arsenic trifluoride
Arsenic pentoxide (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Arsenic pentafluoride is a chemical compound of arsenic and fluorine. The oxidation state of arsenic is +5.
Contents
Synthesis
Arsenic pentafluoride can be prepared by direct combination of arsenic and fluorine[2]:
- 2As + 5F2 → 2AsF5
It can also be prepared by the reaction of arsenic trifluoride and fluorine:
- AsF3 + F2 → AsF5
Properties
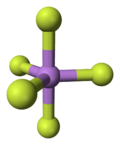
Arsenic pentafluoride is a colourless gas and has a trigonal bipyramidal structure.[2] In the solid state the axial As-F bond lengths are 171.9 pm and the equatorial 166.8 pm.[2]
Reactions
Arsenic pentafluoride forms halide complexes and is a powerful acceptor as shown by the reaction with sulfur tetrafluoride forming an ionic complex.[3]
- AsF5 + SF4 → SF3+ + AsF6−
See also
- List of highly toxic gases
References
- ^ a b c Record of Arsenic(V) fluoride in the GESTIS Substance Database from the IFA, accessed on 24/12/2007
- ^ a b c Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 0080379419.
- ^ An investigation of the structures of the adducts of SF4 with BF3, PF5, AsF5, and SbF5 in the solid state and in solution in HF, M. Azeem, M. Brownstein, and R. J. Gillespie Can. J. Chem. 47(22): 4159–4167 (1969), doi:10.1139/v69-689
Arsenic compounds Categories:- Arsenic compounds
- Fluorides
Wikimedia Foundation. 2010.

