- Dmitri Mendeleev
-
Dmitri Mendeleev 
Dmitri Mendeleev in 1897Born 8 February 1834
Verkhnie Aremzyani, Russian EmpireDied 2 February 1907 (aged 72)
St. Petersburg, Russian EmpireNationality Russian Fields Chemistry, physics and adjacent fields Alma mater Saint Petersburg University Notable students Dmitri Petrovich Konovalov, Valery Gemilian, Alexander Baykov Known for Inventing the Periodic table of chemical elements Dmitri Ivanovich Mendeleev (also romanized Mendeleyev or Mendeleef; Russian: Дми́трий Ива́нович Менделе́ев
 listen (help·info)) (8 February [O.S. 27 January] 1834 – 2 February [O.S. 20 January] 1907), was a Russian chemist and inventor. He is credited as being the creator of the first version of the periodic table of elements. Using the table, he predicted the properties of elements yet to be discovered.
listen (help·info)) (8 February [O.S. 27 January] 1834 – 2 February [O.S. 20 January] 1907), was a Russian chemist and inventor. He is credited as being the creator of the first version of the periodic table of elements. Using the table, he predicted the properties of elements yet to be discovered.Contents
Early Life
Mendeleev was born in the village of Verkhnie Aremzyani, near Tobolsk in Siberia, to Ivan Pavlovich Mendeleev and Maria Dmitrievna Mendeleev (née Kornilieva). His grandfather was Pavel Maximovich Sokolov, a priest of the Russian Orthodox Church from the Tver region.[1] Ivan, along with his brothers and sisters, obtained new family names while attending the theological seminary.[2]
Mendeleev is thought to be the youngest of either 11, 13, 14 or 17 siblings;[3] the exact number differs among sources.[4] His father was a teacher of fine arts, politics and philosophy. Unfortunately for the family's financial well being, his father became blind and lost his teaching position. His mother was forced to work and she restarted her family's abandoned glass factory. At the age of 13, after the passing of his father and the destruction of his mother's factory by fire, Mendeleev attended the Gymnasium in Tobolsk.
In 1849, the now poor Mendeleev family relocated to Saint Petersburg, where he entered the Main Pedagogical Institute in 1850. After graduation, his contraction of tuberculosis caused him to move to the Crimean Peninsula on the northern coast of the Black Sea in 1855. While there he became a science master of the Simferopol gymnasium №1. He returned with fully restored health to Saint Petersburg in 1857.
Later Life
Between 1859 and 1861, he worked on the capillarity of liquids and the workings of the spectroscope in Heidelberg. In late August 1861 he wrote his first book on the spectroscope. On 4 April 1862 he had got engaged to Feozva Nikitichna Leshcheva, and they married on 27 April 1862 at Nikolaev Engineering Institute's church in Saint Petersburg (where he taught).[5] Mendeleev became a professor at the Saint Petersburg Technological Institute and Saint Petersburg State University in 1864 and 1865, respectively. In 1865 he became Doctor of Science for his dissertation "On the Combinations of Water with Alcohol". He achieved tenure in 1867, and by 1871 had transformed Saint Petersburg into an internationally recognized center for chemistry research. In 1876, he became obsessed with Anna Ivanova Popova and began courting her; in 1881 he proposed to her and threatened suicide if she refused. His divorce from Leshcheva was finalized one month after he had married Popova (on 2 April[6]) in early 1882. Even after the divorce, Mendeleev was technically a bigamist; the Russian Orthodox Church required at least seven years before lawful re-marriage. His divorce and the surrounding controversy contributed to his failure to be admitted to the Russian Academy of Sciences (despite his international fame by that time). His daughter from his second marriage, Lyubov, became the wife of the famous Russian poet Alexander Blok. His other children were son Vladimir (a sailor, he took part in the notable Eastern journey of Nicholas II) and daughter Olga, from his first marriage to Feozva, and son Ivan and a pair of twins from Anna.
Though Mendeleev was widely honored by scientific organizations all over Europe, including the Copley Medal from the Royal Society of London, he resigned from Saint Petersburg University on 17 August 1890.
In 1893, he was appointed Director of the Bureau of Weights and Measures. It was in this role that he was directed to formulate new state standards for the production of vodka. As a result of his work, in 1894 new standards for vodka were introduced into Russian law and all vodka had to be produced at 40% alcohol by volume.[7]
Mendeleev also investigated the composition of petroleum, and helped to found the first oil refinery in Russia. He recognized the importance of petroleum as a feedstock for petrochemicals. He is credited with a remark that burning petroleum as a fuel "would be akin to firing up a kitchen stove with bank notes."[8]
In 1905, Mendeleev was elected a member of the Royal Swedish Academy of Sciences. The following year the Nobel Committee for Chemistry recommended to the Swedish Academy to award the Nobel Prize in Chemistry for 1906 to Mendeleev for his discovery of the periodic system. The Chemistry Section of the Swedish Academy supported this recommendation. The Academy was then supposed to approve the Committee choice as it has done in almost every case. Unexpectedly, at the full meeting of the Academy, a dissenting member of the Nobel Committee, Peter Klason, proposed the candidacy of Henri Moissan whom he favored. Svante Arrhenius, although not a member of the Nobel Committee for Chemistry, had a great deal of influence in the Academy and also pressed for the rejection of Mendeleev, arguing that the periodic system was too old to acknowledge its discovery in 1906. According to the contemporaries, Arrhenius was motivated by the grudge he held against Mendeleev for his critique of Arrhenius's dissociation theory. After heated arguments, the majority of the Academy voted for Moissan. The attempts to nominate Mendeleev in 1907 were again frustrated by the absolute opposition of Arrhenius.[9]
In 1907, Mendeleev died at the age of 72 in Saint Petersburg from influenza. The crater Mendeleev on the Moon, as well as element number 101, the radioactive mendelevium, are named after him.
Periodic table
 Sculpture in honor of Mendeleev and the periodic table, located in Bratislava, Slovakia
Sculpture in honor of Mendeleev and the periodic table, located in Bratislava, Slovakia
 Sculpture in Saint Petersburg
Sculpture in Saint Petersburg
Other scientists had suggested in the 1860s that the elements display periodicity. John Newlands published his Law of Octaves in 1865. The lack of spaces for undiscovered elements and the placing of two elements in one box were criticized and his ideas were not accepted. Another was Lothar Meyer, who published a paper in 1864 describing 28 elements. Neither attempted to predict new elements. In 1863 there were 56 known elements with a new element being discovered at a rate of approximately one per year.
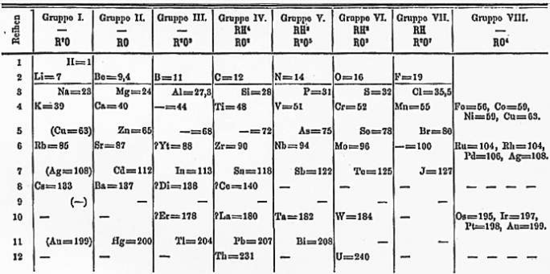
After becoming a teacher, Mendeleev wrote the definitive textbook of his time: Principles of Chemistry (two volumes, 1868–1870). As he attempted to classify the elements according to their chemical properties, he noticed patterns that led him to postulate his periodic table. Mendeleev was unaware of the other work on periodic tables going on in the 1860s. He made the following table, and by adding additional elements following this pattern, developed his extended version of the periodic table.[10][11]
Cl 35.5 K 39 Ca 40 Br 80 Rb 85 Sr 88 I 127 Cs 133 Ba 137 On 6 March 1869, Mendeleev made a formal presentation to the Russian Chemical Society, entitled The Dependence between the Properties of the Atomic Weights of the Elements, which described elements according to both atomic weight and valence. This presentation stated that
- The elements, if arranged according to their atomic weight, exhibit an apparent periodicity of properties.
- Elements which are similar in regards to their chemical properties have atomic weights which are either of nearly the same value (e.g., Pt, Ir, Os) or which increase regularly (e.g., K, Rb, Cs).
- The arrangement of the elements in groups of elements in the order of their atomic weights corresponds to their so-called valencies, as well as, to some extent, to their distinctive chemical properties; as is apparent among other series in that of Li, Be, B, C, N, O, and F.
- The elements which are the most widely diffused have small atomic weights.
- The magnitude of the atomic weight determines the character of the element, just as the magnitude of the molecule determines the character of a compound body.
- We must expect the discovery of many yet unknown elements–for example, two elements, analogous to aluminium and silicon, whose atomic weights would be between 65 and 75.
- The atomic weight of an element may sometimes be amended by a knowledge of those of its contiguous elements. Thus the atomic weight of tellurium must lie between 123 and 126, and cannot be 128. Here Mendeleev seems to be wrong as the "atomic mass" of tellurium (127.6) remains higher than that of iodine (126.9) as displayed on modern periodic tables, but this is due to the way atomic masses are calculated, based on a weighted average of all of an element's common isotopes, not just the one-to-one proton/neutron-ratio version of the element to which Mendeleev was referring.
- Certain characteristic properties of elements can be foretold from their atomic weights.
Mendeleev published his periodic table of all known elements and predicted several new elements to complete the table. Only a few months after, Meyer published a virtually identical table. Some consider Meyer and Mendeleev the co-creators of the periodic table, but virtually everybody[who?] agrees that Mendeleev's accurate prediction of the qualities of what he called ekasilicon, ekaaluminium and ekaboron (germanium, gallium and scandium, respectively) qualifies him for the majority of the credit for the table.
For his predicted eight elements, he used the prefixes of eka, dvi, and tri (Sanskrit one, two, three) in their naming. Mendeleev questioned some of the currently accepted atomic weights (they could be measured only with a relatively low accuracy at that time), pointing out that they did not correspond to those suggested by his Periodic Law. He noted that tellurium has a higher atomic weight than iodine, but he placed them in the right order, incorrectly predicting that the accepted atomic weights at the time were at fault. He was puzzled about where to put the known lanthanides, and predicted the existence of another row to the table which were the actinides which were some of the heaviest in atomic mass. Some people dismissed Mendeleev for predicting that there would be more elements, but he was proven to be correct when Ga (gallium) and Ge (germanium) were found in 1875 and 1886 respectively, fitting perfectly into the two missing spaces.[12]
By giving Sanskrit names to his "missing" elements, Mendeleev showed his appreciation and debt to the Sanskrit grammarians of ancient India, who had created sophisticated theories of language based on their discovery of the two-dimensional patterns in basic sounds. According to Professor Paul Kiparsky of Stanford University, Mendeleev was a friend and colleague of the Sanskritist Böhtlingk, who was preparing the second edition of his book on Pāṇini[13] at about this time, and Mendeleev wished to honor Pāṇini with his nomenclature.[14] Noting that there are striking similarities between the periodic table and the introductory Śiva Sūtras in Pāṇini's grammar, Prof. Kiparsky says:
[T]he analogies between the two systems are striking. Just as Panini found that the phonological patterning of sounds in the language is a function of their articulatory properties, so Mendeleev found that the chemical properties of elements are a function of their atomic weights. Like Panini, Mendeleev arrived at his discovery through a search for the "grammar" of the elements...[15]
Other achievements
Mendeleev made other important contributions to chemistry. The Russian chemist and science historian Lev Chugaev has characterized him as "a chemist of genius, first-class physicist, a fruitful researcher in the fields of hydrodynamics, meteorology, geology, certain branches of chemical technology (explosives, petroleum, and fuels, for example) and other disciplines adjacent to chemistry and physics, a thorough expert of chemical industry and industry in general, and an original thinker in the field of economy." Mendeleev was one of the founders, in 1869, of the Russian Chemical Society. He worked on the theory and practice of protectionist trade and on agriculture.
In an attempt at a chemical conception of the Aether, he put forward a hypothesis that there existed two inert chemical elements of lesser atomic weight than hydrogen. Of these two proposed elements, he thought the lighter to be an all-penetrating, all-pervasive gas, and the slightly heavier one to be a proposed element, coronium.
Mendeleev devoted much study and made important contributions to the determination of the nature of such indefinite compounds as solutions.
In another department of physical chemistry, he investigated the expansion of liquids with heat, and devised a formula similar to Gay-Lussac's law of the uniformity of the expansion of gases, while in 1861 he anticipated Thomas Andrews' conception of the critical temperature of gases by defining the absolute boiling-point of a substance as the temperature at which cohesion and heat of vaporization become equal to zero and the liquid changes to vapor, irrespective of the pressure and volume.
Mendeleev is given credit for the introduction of the metric system to the Russian Empire.
He invented pyrocollodion, a kind of smokeless powder based on nitrocellulose. This work had been commissioned by the Russian Navy, which however did not adopt its use. In 1892 Mendeleev organized its manufacture.
Mendeleev studied petroleum origin and concluded hydrocarbons are abiogenic and form deep within the earth – see Abiogenic petroleum origin. He wrote: "The capital fact to note is that petroleum was born in the depths of the earth, and it is only there that we must seek its origin." (Dmitri Mendeleev, 1877)[16]
See also
- Mendeleev's predicted elements
- Periodic Systems of Small Molecules
- List of Russian chemists
References
- ^ Dmitriy Mendeleev: A Short CV, and A Story of Life, mendcomm.org
- ^ Удомельские корни Дмитрия Ивановича Менделеева (1834–1907), starina.library.tver.ru
- ^ Johnson, George (3 January 2006). "The Nitpicking of the Masses vs. the Authority of the Experts". New York Times. http://www.nytimes.com/2006/01/03/science/03comm.html. Retrieved 14 March 2011.
- ^ The number of Mendeleev's siblings is a matter of some historical dispute. When the Princeton historian of science Michael Gordin reviewed this article as part of an analysis of the accuracy of Wikipedia for the 14 December 2005 issue of Nature, he cited as one of Wikipedia's errors that "They say Mendeleev is the 14th child. He is the 14th surviving child of 17 total. 14 is right out." However in a New York Times article from January 2006, it was noted that in Gordin's own 2004 biography of Mendeleev, he also had the Russian chemist listed as the 17th child, and quoted Gordin's response to this as being: "That's curious. I believe that is a typographical error in my book. Mendeleyev was the final child, that is certain, and the number the reliable sources have is 13." Gordin's book specifically says that Mendeleev's mother bore her husband "seventeen children, of whom eight survived to young adulthood," with Mendeleev being the youngest. See: George Johnson, "The Nitpicking of the Masses vs. the Authority of the Experts" New York Times (3 January 2006) (online); "Supplementary information to accompany Nature news article 'Internet encyclopaedias go head to head' (Nature 438, 900–901; 2005)", posted on the Nature website 22 December 2005 (PDF); Gordin 2004, p. 178.
- ^ "Rustest.spb.ru". Rustest.spb.ru. http://www.rustest.spb.ru/site/9/4/index.html. Retrieved 13 March 2010.
- ^ "Gazeta.ua". Gazeta.ua. 9 March 2010. http://gazeta.ua/index.php?id=157545&lang=ru. Retrieved 13 March 2010.
- ^ RussianFoods.com. "RussianFoods.com". RussianFoods.com. http://www.russianfoods.com/cuisine/article00016/default.asp. Retrieved 13 March 2010.
- ^ John W. Moore, Conrad L. Stanitski, Peter C. Jurs. "Chemistry: The Molecular Science, Volume 1". http://books.google.com/books?id=BO9ocnho9P4C&lpg=PA546&pg=PA546#v=onepage&q&f=false. Retrieved 6 September 2011.
- ^ Friedman, Robert M. (2001). The politics of excellence: behind the Nobel Prize in science. New York: Times Books. pp. 32–34. ISBN 0-7167-3103-7.
- ^ A brief history of the development of the period table, wou.edu
- ^ Mendeleev and the Periodic Table, chemsheets.co.uk
- ^ Emsley, John (2001). Nature's Building Blocks ((Hardcover, First Edition) ed.). Oxford University Press. pp. 521–522. ISBN 0198503407.
- ^ Otto Böhtlingk, Panini's Grammatik: Herausgegeben, Ubersetzt, Erlautert und MIT Verschiedenen Indices Versehe. St. Petersburg, 1839–40.
- ^ Kiparsky, Paul. "Economy and the construction of the Sivasutras." In M. M. Deshpande and S. Bhate (eds.), Paninian Studies. Ann Arbor, Michigan, 1991.
- ^ Kak, Subhash (2004). "Mendeleev and the Periodic Table of Elements". Sandhan 4 (2): 115–123. arXiv:physics/0411080
- ^ Mendeleev, D., 1877. L'Origine du pétrole. Revue Scientifique, 2e Ser., VIII, p. 409-416.
Further reading
- Gordin, Michael (2004). A Well-Ordered Thing: Dmitrii Mendeleev and the Shadow of the Periodic Table. New York: Basic Books. ISBN 0-465-02775-X.
- Mendeleyev, Dmitry Ivanovich; Jensen, William B. (2005). Mendeleev on the Periodic Law: Selected Writings, 1869 – 1905. Mineola, New York: Dover Publications. ISBN 0-486-44571-2.
- Strathern, Paul (2001). Mendeleyev's Dream: The Quest For the Elements. New York: St Martins Press. ISBN 024114065X.
- Mendeleev, Dmitrii Ivanovich (1901). Principles of Chemistry. New York: Collier. http://www.archive.org/details/principlesofchem00menduoft.
External links
Biographies
- Roger Rumppe and Michael E. Sixtus, "Ich bin Mendelejeff", care of the Woodrow Wilson Leadership Program in Chemistry. 20 sources. Notes, among other things, that various sources list D.M.'s siblings as being 10 to 16 in number. woodrow.org
Periodic table
- Original Periodic Table, annotated. webserver.lemoyne.edu
- Mendeleev's first draft version of the Periodic Table, 17 February 1869. chemheritage.org
Other
- References about Mendeleev, maintained by Eugene V. Babaev, last updated May 2005 (as of December 2005). chem.msu.su
- Faraday Lecture by Mendeleev, 4 July 1889, annotated. webserver.lemoyne.edu
- Mendeleev and Sanskrit, uk.arxiv.org
- Picture of Mendeleev, Edgar Fahs Smith Collection, University of Pennsylvania, dewey.library.upenn.edu
- Everything in its Place, nytimes.com
- Mendeleev profile at thinkquest.org
- Dmitri Ivanovich Mendeleev article on h2g2, bbc.co.uk.
- Who was Dmitri Mendeleev?, chemistry.co.nz
Categories:- 1834 births
- 1907 deaths
- Demidov Prize laureates
- Eastern Orthodox Christians from Russia
- Faraday Lecturers
- Inorganic chemists
- Members of the Prussian Academy of Sciences
- Members of the Royal Swedish Academy of Sciences
- Members of the Russian Academy of Sciences
- People from Tyumen Oblast
- Recipients of the Copley Medal
- Russian Orthodox Christians
- Russian chemists
- Russian inventors
- Russian monarchists
- Russian scientists
- Saint Petersburg State University alumni
- Saint Petersburg Technological Institute alumni
- Foreign Members of the Royal Society
- Members of the Serbian Academy of Sciences and Arts
Wikimedia Foundation. 2010.