- Galvanic corrosion
-
Galvanic corrosion is an electrochemical process in which one metal corrodes preferentially to another when both metals are in electrical contact and immersed in an electrolyte. The same galvanic reaction is exploited in primary batteries to generate a voltage.
Contents
Overview
Dissimilar metals and alloys have different electrode potentials and when two or more come into contact in an electrolyte a galvanic couple is set up. The potenetial difference between the dissimilar metals is the driving force for the accelerated attack on the anode member of the galvanic couple.
The electrolyte provides a means for ion migration whereby metallic ions can move from the anode to the cathode. This leads to the anodic metal corroding more quickly than it otherwise would; the corrosion of the cathodic metal is retarded even to the point of stopping. The presence of an electrolyte and a electronic conducting path between the metals is essential for galvanic corrosion to occur.
In some cases, this reaction is intentionally encouraged. For example, low-cost household batteries typically contain carbon-zinc cells. As part of a closed circuit (the electron pathway), the zinc within the cell will corrode preferentially (the ion pathway). Another example is the cathodic protection of buried or submerged structures. In this example, sacrificial anodes work as part of a galvanic couple, promoting corrosion of the anode, rather than the protected subject metal.
In other cases, such as HVAC systems with bimetallic plumbing/piping (most commonly copper and cast iron) galvanic corrosion will contribute to accelerated corrosion rates of the system. Corrosion inhibitors such as sodium nitrite or sodium molybdate can be introduced to these systems to reduce the galvanic potential. However, the application of these corrosion inhibitors must be monitored closely. If the application of corrosion inhibitors increases the conductivity of the water within the system, the galvanic corrosion potential can be exponentially increased. pH is also a major consideration with regards to closed loop bimetallic circulating systems. Should the pH and corrosion inhibition doses be incorrect, galvanic corrosion will be accelerated. In most HVAC systems, the concept of anodes and cathodes are not an option as they would need to be applied within the plumbing of the system and, over time, would corrode and cause potential mechanical damage to circulating pumps, heat exchangers, etc.[1]
Galvanic series
Metals (and their alloys) can be arranged in a galvanic series representing the potential they develop in a given electrolyte against a standard reference electrode. The relative position of two metals on such a series gives a good indication of which metal is more likely to corrode more quickly. However, other factors such as water aeration and flow rate can influence the process markedly.
Galvanic corrosion is of major interest to the marine industry. Galvanic series tables for seawater are commonplace due to the extensive use of metal in shipbuilding. It is possible that corrosion of silver brazing in a salt water pipe caused a failure that led to the USS Thresher sinking with all men lost.
The common technique of cleaning silver by immersion of the silver and a piece of aluminium in a salt water bath (usually sodium bicarbonate) is an example of galvanic corrosion. (Care should be exercised for reasons such as this will strip silver oxide from the silver which may be there for decoration. Use on plated silver is inadvisable as this may introduce unwanted galvanic corrosion with the base metal.)
Preventing galvanic corrosion
There are several ways of reducing and preventing this form of corrosion.
- One way is to electrically insulate the two metals from each other. Unless they are in electrical contact, there can be no galvanic couple set up. This can be done using plastic or another insulator to separate steel water pipes from copper-based fittings or by using a coat of grease to separate aluminium and steel parts. Use of absorbent washers that may retain fluid is often counter-productive. Piping can be isolated with a spool of pipe made of plastic materials or made of metal material internally coated or lined. It is important that the spool has a minimum length of approx 500 & nbsp; mm to be effective.
- Another way is to keep the metals dry and/or shielded from ionic compounds (salts, acids, bases), for example by painting or encasing the protected metal in plastic or epoxy, and allowing them to dry.
- Coating the two materials or if it is not possible to coat both, the coating shall be applied to the more noble, the material with higher potential. This is necessary because if the coating is applied only on the more active material, in case of damage of the coating there will be a large cathode area and a very small anode area, and for the area effect the corrosion rate will be very high.
- It is also possible to choose metals that have similar potentials. The more closely matched the individual potentials, the lesser the potential difference and hence the lesser the galvanic current. Using the same metal for all construction is the most precise way of matching potentials.
Electroplating or other plating can also help. This tends to use more noble metals that resist corrosion better. Chrome, nickel, silver and gold can all be used.
Cathodic protection uses one or more sacrificial anodes made of a metal which is more active than the protected metal. Metals commonly used for sacrificial anodes include zinc, magnesium, and aluminium. This is commonplace in water heaters. Failure to regularly replace sacrificial anodes in water heaters severely diminishes the lifetime of the tank.
Finally, an electrical power supply may be connected to oppose the corrosive galvanic current. (see impressed current cathodic protection)
For example, consider a system is composed of 316 SS (a 300 series stainless steel; it is a very noble alloy meaning it is quite resistant to corrosion and has a high potential) and a mild steel (a very active metal with lower potential). The mild steel will corrode in the presence of an electrolyte such as salt water. If a sacrificial anode is used (such as a zinc alloy, aluminium alloy, or magnesium), these anodes will corrode, protecting the other metals. This is a common practice in the marine industry to protect ship equipment. Boats and vessels that are in salt water use either zinc alloy or aluminium alloy. If boats are only in fresh water, a magnesium alloy is used. Magnesium has one of the highest galvanic potentials of any metal. If it is used in a salt water application on a steel or aluminium hull boat, hydrogen bubbles will form under the paint, causing blistering and peeling.
Metal boats connected to a mains shore line will normally have to have the hull connected to earth for safety reasons. However the end of that earth connection is likely to be a copper rod buried within the marina, resulting in a steel-copper "battery" of about 0.5V. For such cases the use of a galvanic isolator is essential - typically 2 diodes in series, preventing any current flow while the applied voltage is less than 1.4V (i.e. 0.7V per diode), but allowing a full flow in case of an earth fault. It has been noted that there will still be a very minor leak through the diodes which may result in slightly faster corrosion than normal.
Examples
A common example of galvanic corrosion is the rusting of corrugated iron sheet, which becomes widespread when the protective zinc coating is broken and the underlying steel is attacked. The zinc is attacked preferentially because it is less noble, but when consumed, rusting will occur in earnest. With a tin can, the opposite is true because the tin is more noble than the underlying steel, so when the coating is broken, the steel is attacked preferentially.
Statue of Liberty
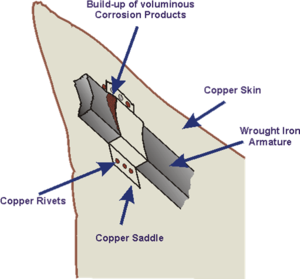
A rather more spectacular example occurred in the Statue of Liberty when regular maintenance in the 1980s showed that galvanic corrosion had taken place between the outer copper skin and the wrought iron support structure.
Although the problem had been anticipated when the structure was built by Gustave Eiffel to Frédéric Bartholdi's design in the 1880s, the insulation of shellac between the two metals failed over a period of time and resulted in rusting of the iron supports. The renovation replaced the original insulation with PTFE. The structure was far from unsafe owing to the large number of unaffected connections, but it was regarded as a precautionary measure for what is considered a national symbol of the United States.[2][3]
In 17th-century England, Samuel Pepys then serving as Admiralty Secretary, agreed to the removal of lead sheathing on British Royal Navy vessels to prevent the mysterious disintegration of their rudder-irons and bolt-heads—though he confessed himself baffled as to the reason the lead caused the corrosion.[4]
The problem recurred when vessels were sheathed in copper. The protective copper on the Royal Navy frigate HMS Alarm detached from the wooden hull in many places because the iron nails which had been used to fasten the copper to the timbers had been "much rotted." Closer inspection revealed that some nails, which were less corroded, were insulated from the copper by brown paper which was trapped under the nail head. The copper had been delivered to the dockyard wrapped in the paper which was not removed before the sheets were nailed to the hull. The obvious conclusion therefore, and the one which was contained in a report to the Admiralty of 1763, was that iron should not be allowed direct contact with copper in sea water to avoid severe corrosion. Later ships were designed with this in mind. Not only was sea water a very good electrolyte owing to its high salt concentration, but attack of the nails was encouraged by their very small exposed area compared with that of the copper-sheathed hull.[5][6]
Lasagna cell
A "lasagna cell" is accidentally produced when salty food such as lasagna is stored in a steel baking pan and is covered with aluminium foil. After a few hours the foil develops small holes where it touches the lasagna, and the food surface becomes covered with small spots composed of corroded aluminium.[7]
In this example, the salty food (e.g. lasagna) is the electrolyte, the aluminium foil the anode and the steel pan the cathode. If the aluminium foil only touches the electrolyte in small areas, the galvanic corrosion is concentrated and corrosion can occur fairly rapidly.
Galvanic compatibility
Often when design requires that dissimilar metals come in contact, the galvanic compatibility is managed by finishes and plating. The finishing and plating selected facilitate the dissimilar materials being in contact and protect the base materials from corrosion.[8][page needed]
Harsh environments, such as outdoors, high humidity, and salt environments, fall into this category. Typically there should be not more than 0.15 V difference in the "Anodic Index". For example; gold - silver would have a difference of 0.15V being acceptable. For normal environments, such as storage in warehouses or non-temperature and humidity controlled environments, there should not be more than 0.25 V difference in the "Anodic Index". For controlled environments, in which temperature and humidity are controlled, 0.50 V can be tolerated.[8][page needed]
Anodic index[8][page needed] Metal Index (V) Most Cathodic Gold, solid and plated, Gold-platinum alloy 0.00 Rhodium plated on silver-plated copper 0.05 Silver, solid or plated; monel metal. High nickel-copper alloys 0.15 Nickel, solid or plated, titanium an s alloys, Monel 0.30 Copper, solid or plated; low brasses or bronzes; silver solder; German silvery high copper-nickel alloys; nickel-chromium alloys 0.35 Brass and bronzes 0.40 High brasses and bronzes 0.45 18% chromium type corrosion-resistant steels 0.50 Chromium plated; tin plated; 12% chromium type corrosion-resistant steels 0.60 Tin-plate; tin-lead solder 0.65 Lead, solid or plated; high lead alloys 0.70 2000 series wrought aluminum 0.75 Iron, wrought, gray or malleable, plain carbon and low alloy steels 0.85 Aluminum, wrought alloys other than 2000 series aluminum, cast alloys of the silicon type 0.90 Aluminum, cast alloys other than silicon type, cadmium, plated and chromate 0.95 Hot-dip-zinc plate; galvanized steel 1.20 Zinc, wrought; zinc-base die-casting alloys; zinc plated 1.25 Magnesium & magnesium-base alloys, cast or wrought 1.75 Beryllium 1.85 Most Anodic See also
- Corrosion
- Galvanising
- Galvanic anode
References
- ^ M. Houser, Corrosion Control Services, Inc., introduction handbook
- ^ Corrosion Doctors [1] (Retrieved January 2011)
- ^ Copper.org [2] (Retrieved January 2011)
- ^ Bryant, Arthur (1935). Samuel Pepys: The Years of Peril. Cambridge: Macmillan. p. 370.
- ^ CLI Houston [3] (Retrieved January 2011)
- ^ Corrosion Doctors [4] (Retrieved January 2011)
- ^ Water. Hemat, R.A.S. Editor: Urotext. ISBN 1-903737-12-5. p. 826
- ^ a b c Handbook of Corrosion Engineering by Pierre R. Roberge
External links
- Galvanic Corrosion and Other Types of Corrosion
- Galvanic corrosion explained
- Corrosion Doctors
- Galvanic Corrosion Theory and documents
- Galvanic series
- Electrochemistry of corrosion From the Yeager Center at CWRU.
- Bimetallic corrosion
- The Straight Dope: why does ketchup dissolve aluminium?
- PIRA physics lecture demonstration 5e40.25
- Cathodic Protection 101: A basic tutorial
Categories:
Wikimedia Foundation. 2010.