- Strontium titanate
-
Strontium titanate  Systematic nameStrontium(2+) oxotitaniumbis(olate)[citation needed]Other namesStrontium titanium oxide
Systematic nameStrontium(2+) oxotitaniumbis(olate)[citation needed]Other namesStrontium titanium oxide
TausoniteIdentifiers CAS number 12060-59-2 
PubChem 82899 ChemSpider 74801 
EC number 235-044-1 MeSH Strontium+titanium+oxide Jmol-3D images Image 1
Image 2- [Sr++].[O-][Ti]([O-])=O
[Sr+2].[O-][Ti]([O-])=O
Properties Molecular formula SrTiO3 Molar mass 183.49 g mol-1 Exact mass 183.838305258 g mol-1 Appearance White, opaque crystals Density 4.81 g cm-3 Melting point 2060 °C, 2333 K, 3740 °F
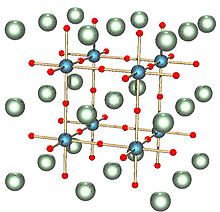
Refractive index (nD) 2.41 Structure Crystal structure Simple cubic  titanate (verify) (what is:
titanate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Strontium titanate is an oxide of strontium and titanium with the chemical formula SrTiO3. At room temperature, it is a centrosymmetric paraelectric material with a perovskite structure. At low temperatures it approaches a ferroelectric phase transition with a very large dielectric constant ~104 but remains paraelectric down to the lowest temperatures measured as a result of quantum fluctuations.[1] It was long thought to be a wholly artificial material, until 1982 when its natural counterpart—discovered in Siberia and named tausonite—was recognised by the IMA. Tausonite remains an extremely rare mineral in nature, occurring as very tiny crystals. Its most important application has been in its synthesized form wherein it is occasionally encountered as a diamond simulant, in precision optics, in varistors, and in advanced ceramics.
The name tausonite was given in honour of Lev Vladimirovich Tauson (1917–1989), a Russian geochemist. Disused trade names for the synthetic product include strontium mesotitanate, Fabulite, Diagem, and Marvelite. Other than its type locality of the Murun Massif in the Sakha Republic, natural tausonite is also found in Cerro Sarambi, Concepción department, Paraguay; and along the Kotaki River of Honshū, Japan.[2][3]
Contents
Properties
Strontium titanate is both much denser (specific gravity 4.88 for natural, 5.13 for synthetic) and much softer (Mohs hardness 6–6.5 for natural, 5.5 for synthetic) than diamond. Its crystal system is cubic and its refractive index (2.41—as measured by sodium light, 589.3 nm) is nearly identical to that of diamond, but the dispersion (the optical property responsible for the "fire" of the cut stones) of strontium titanate is over four times higher, at 0.19 (B–G interval). This results in an excess of fire when compared to diamond.[2][3]
Synthetics are usually transparent and colourless, but can be doped with certain rare earth or transition metals to give reds, yellows, browns, and blues. Natural tausonite is usually translucent to opaque, in shades of reddish brown, dark red, or grey. Both have an adamantine (diamond-like) lustre. Strontium titanate is considered extremely brittle with a conchoidal fracture; natural material is cubic or octahedral in habit and streaks brown. Through a hand-held (direct vision) spectroscope, doped synthetics will exhibit a rich absorption spectrum typical of doped stones. Synthetic material has a melting point of ca. 2080°C (3776°F) and is readily attacked by hydrofluoric acid.[2][3]
The synthetic material has a very large dielectric constant (300) at room temperature and low electric field. It is also used in high-voltage capacitors. Strontium titanate becomes superconducting below 0.35 K and was the first insulator and oxide discovered to be superconductive.[4]
At temperatures lower than 105 K, its cubic structure transforms to tetragonal.[5] It is an excellent substrate for epitaxial growth of high-temperature superconductors and many oxide-based thin films. Its monocrystals can be used as optical windows and high-quality sputter deposition targets.
SrTiO3 is a suitable material for electronics: niobium-doped strontium titanate, is electrically conductive. High-quality, epitaxial SrTiO3 layers can also be grown on silicon without forming silicon dioxide, thereby making SrTiO3 an alternative gate dielectric material. This also enables the integration of other thin film perovskite oxides onto silicon.[6]
Synthesis
Synthetic strontium titanate was one of several titanates patented during the late 1940s and early 1950s; other titanates included barium titanate and calcium titanate. Research was conducted primarily at the National Lead Company (later renamed N. L. Industries, Inc.) in the United States, by Leon Merker and Langtry E. Lynd. Merker and Lynd first patented the growth process on February 10, 1953; a number of refinements were subsequently patented over the next four years, such as modifications to the feed powder and additions of colouring dopants.
A modification to the basic Verneuil process (also known as flame-fusion) is the favoured method of growth. An inverted oxy-hydrogen blowpipe is used, with feed powder mixed with oxygen carefully fed through the blowpipe in the typical fashion, but with the addition of a third pipe to deliver oxygen—creating a tricone burner. The extra oxygen is required for successful formation of strontium titanate, which would otherwise fail to oxidize completely due to the titanium component. The ratio is ca. 1.5 volumes of hydrogen for each volume of oxygen. The highly purified feed powder is derived by first producing titanyl double oxalate salt (SrTiO(C2O4)2·2H2O) by reacting strontium chloride (SrCl2) and oxalic acid ((COOH)2.2H2O) with titanium tetrachloride (TiCl4). The salt is washed to completely eliminate chloride, heated to 1000°C in order to produce a free-flowing granular powder of the required composition, and is then ground and sieved to ensure all particles are between 0.2–0.5 micrometres in size.[7]
The feed powder falls through the oxyhydrogen flame, melts, and lands on a rotating and slowly descending pedestal below. The height of the pedestal is constantly adjusted to keep its top at the optimal position below the flame, and over a number of hours the molten powder cools and crystallises to form a single pedunculated pear or boule crystal. This boule is usually no larger than 2.5 centimetres in diameter and 10 centimetres long; it is an opaque black to begin with, requiring further annealing in an oxidizing atmosphere in order to make the crystal colourless and to relieve strain. This is done at over 1000°C for 12 hours.[7]
Use as a diamond simulant
Its cubic structure and high dispersion once made synthetic strontium titanate a prime candidate for simulating diamond. Beginning ca. 1955, large quantities of strontium titanate were manufactured for this sole purpose. Strontium titanate was in competition with synthetic rutile ("titania") at the time, and had the advantage of lacking the unfortunate yellow tinge and strong birefringence inherent to the latter material. While it was softer, it was significantly closer to diamond in likeness. Eventually, however, both would fall into disuse, being eclipsed by the creation of "better" simulants: first by yttrium aluminium garnet (YAG) and followed shortly after by gadolinium gallium garnet (GGG); and finally by the (to date) ultimate simulant in terms of diamond-likeness and cost-effectiveness, cubic zirconia.[8]
Despite being outmoded, strontium titanate is still manufactured and periodically encountered in jewellery. It is one of the most costly of diamond simulants, and due to its rarity collectors may pay a premium for large i.e. >2 carat (400 mg) specimens. As a diamond simulant, strontium titanate is most deceptive when mingled with melée i.e. <0.20 carat (40 mg) stones and when it is used as the base material for a composite or doublet stone (with, e.g., synthetic corundum as the crown or top of the stone). Under the microscope, gemmologists distinguish strontium titanate from diamond by the former's softness—manifested by surface abrasions—and excess dispersion (to the trained eye), and occasional gas bubbles which are remnants of synthesis. Doublets can be detected by a join line at the girdle ("waist" of the stone) and flattened air bubbles or glue visible within the stone at the point of bonding.[9][10][11]
References
- ^ K. A. Muller and H. Burkard (1979). "SrTiO3: An intrinsic quantum paraelectric below 4 K". Phys. Rev. B 19: 3593–3602. doi:10.1103/PhysRevB.19.3593.
- ^ a b c "Tausonite". Webmineral.. http://webmineral.com/data/Tausonite.shtml. Retrieved 2009-06-06.
- ^ a b c "Tausonite". Mindat. http://www.mindat.org/min-3895.html. Retrieved 2009-06-06.
- ^ C. S. Koonce et al. (1967). "Superconducting Transition Temperatures of Semiconducting SrTiO3". Phys. Rev. 163: 380. doi:10.1103/PhysRev.163.380.
- ^ L. Rimai and G. A. deMars (1962). "Electron Paramagnetic Resonance of Trivalent Gadolinium Ions in Strontium and Barium Titanates". Phys. Rev. 127: 702. doi:10.1103/PhysRev.127.702.
- ^ R. A. McKee, F. J. Walker, and M. F. Chisholm (1998). "Crystalline Oxides on Silicon: The First Five Monolayers". Phys. Rev. Lett. 81: 3014. Bibcode 1998PhRvL..81.3014M. doi:10.1103/PhysRevLett.81.3014.
- ^ a b H. J. Scheel and P. Capper (2008). Crystal growth technology: from fundamentals and simulation to large-scale production. Wiley-VCH. p. 431. ISBN 3527317627.
- ^ R. W. Hesse (2007). Jewelrymaking through history: an encyclopedia. Greenwood Publishing Group. p. 73. ISBN 0313335079.
- ^ Nassau, K. (1980). Gems made by man. Santa Monica, California: Gemological Institute of America. pp. 214–221. ISBN 0873110161.
- ^ O'Donoghue, M. (2002). Synthetic, imitation & treated gemstones. Great Britain: Elsevier Butterworth-Heinemann. pp. 34, 65. ISBN 0750631732.
- ^ Read, P. G. (1999). Gemmology, second edition. Great Britain: Butterworth-Heinemann. pp. 173, 176, 177, 293. ISBN 0-7506-4411-7.
External links
Strontium compounds Categories:- Titanates
- Strontium compounds
- Gemstones
- Oxide minerals
- Ceramic materials
- Synthetic minerals
- Transition metal oxides
- Diamond simulants
- [Sr++].[O-][Ti]([O-])=O
Wikimedia Foundation. 2010.