- Neon lamp
-
See also: Neon lightingA General electric NE-34 glow lamp, manufactured circa 1930.

A neon lamp (also neon glow lamp) is a miniature gas discharge lamp that typically contains neon gas at a low pressure in a glass capsule. Only a thin region adjacent to the electrodes glows in these lamps, which distinguishes them from the much longer and brighter neon tubes used for signage. The term "neon lamp" is generally extended to lamps with similar design that operate with different gases. Neon glow lamps were very common in the displays of electronic instruments through the 1970s; the basic design of neon lamps is now incorporated in contemporary plasma displays.
Contents
History
Neon was discovered in 1898 by William Ramsey and Morris W. Travers. The characteristic, brilliant red color that is emitted by gaseous neon when excited electrically was noted immediately; Travers later wrote, "the blaze of crimson light from the tube told its own story and was a sight to dwell upon and never forget."[1]
Neon's scarcity precluded its prompt application for electrical lighting along the lines of Moore tubes, which used electric discharges in nitrogen and which were commercialized in the early 1900s. After 1902, Georges Claude's company, Air Liquide, was producing industrial quantities of neon as a byproduct of his air liquefaction business, and in December 1910 Claude demonstrated modern neon lighting based on a sealed tube of neon. In 1915 a U.S. patent was issued to Claude covering the design of the electrodes for neon tube lights;[2] this patent became the basis for the monopoly held in the U.S. by his company, Claude Neon Lights, through the early 1930s.[3]
Around 1917, Daniel McFarlan Moore, then working at the General Electric Company, developed the neon lamp, which has a very different design than the much larger neon tubes used for neon lighting. The difference in design was sufficient that a U.S. patent was issued for the lamp in 1919.[4] A Smithsonian Institution website notes, "These small, low power devices use a physical principle called coronal discharge. Moore mounted two electrodes close together in a bulb and added neon or argon gas. The electrodes would glow brightly in red or blue, depending on the gas, and the lamps lasted for years. Since the electrodes could take almost any shape imaginable, a popular application has been fanciful decorative lamps. Glow lamps found practical use as indicators in instrument panels and in many home appliances until the widespread commercialisation of Light-Emitting Diodes (LEDs) in the 1970s."[5]
Description
A small electric current, which may be AC or DC, is allowed through the tube, causing it to glow orange-red. The formulation of the gas is typically the classic Penning mixture, 99.5% neon and 0.5% argon, which has lower striking voltage than pure neon. The applied voltage must initially reach the striking voltage before the lamp is visible. At the striking voltage, the lamp enters a breakdown mode and exhibits a glow discharge. Once lit, the voltage required to sustain the discharge is significantly (~30%) lower than the striking voltage. This is due to the organization of positive ions near the cathode. When driven from a DC source, only the negatively charged electrode (cathode) will glow. When driven from an AC source, both electrodes will glow (each during alternate half cycles). These attributes make neon bulbs (with series resistors) a convenient low-cost voltage testers; they determine whether a given voltage source is AC or DC, and if DC, the polarity of the points being tested. Neon lamps operate using a low current glow discharge. Higher power devices, such as mercury-vapor lamps or metal halide lamps use a higher current arc discharge.
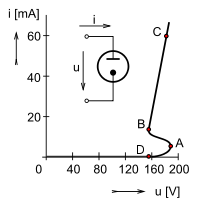 Graph showing the relationship between current and voltage across a neon lamp.[dubious ]
Graph showing the relationship between current and voltage across a neon lamp.[dubious ]
Once the neon lamp has reached breakdown, it can support a large current flow. Because of this characteristic, electrical circuitry external to the neon lamp must limit the current through the circuit or else the current will rapidly increase until the lamp is destroyed. For indicator-sized lamps, a resistor typically limits the current. Larger neon sign sized lamps often use a specially constructed high voltage transformer with high leakage inductance or other electrical ballast to limit the available current.
When the current through the lamp is lower than the current for the highest-current discharge path, the glow discharge may become unstable and not cover the entire surface of the electrodes.[dubious ] This may be a sign of aging of the indicator bulb, and is exploited in the decorative "flicker flame" neon lamps. However, while too low a current causes flickering, too high a current increases the wear of the electrodes by stimulating sputtering, which coats the internal surface of the lamp with metal and causes it to darken.
The potential needed to strike the discharge is higher than what is needed to sustain the discharge. When there is not enough current, the glow forms around only part of the electrode surface. Convective currents make the glowing areas flow upwards, not unlike the discharge in a Jacob's ladder. A photoionization effect can also be observed here, as the electrode area covered by the glow discharge can be increased by shining light at the lamp.
In comparison with incandescent light bulbs, neon lamps have much higher luminous efficacy. Incandescence is heat-driven light emission, so a large portion of the electric energy put into an incandescent bulb is converted into heat. Non-incandescent light sources such as neon light bulbs, fluorescent light bulbs, and light emitting diodes are therefore much more energy efficient than normal incandescent light bulbs. Green neon[clarification needed] bulbs can produce up to 65 lumens per watt of power input, while white neon bulbs have an efficacy of around 50 lumens per watt. In contrast, a standard incandescent light bulb only produces around 13.5 lumens per watt.[6]
Applications
 The digits of a Nixie tube.
The digits of a Nixie tube.
Most small neon (indicator-sized) lamps, such as the common NE-2, break down at between 90 and 110 volts. This feature enables their use as very simple voltage regulators or overvoltage protection devices. In the 1960s General Electric (GE), Signalite, and other firms made special extra-stable neon lamps for electronic uses. They even devised digital logic circuits, binary memories, and frequency dividers using neon lamps.[7][8][9][10][11][12][13][14] Such circuits appeared in electronic organs of the 1950s, as well as some instrumentation. At least some of these lamps had a glow concentrated into a small spot on the cathode, which made them unsuited to use as indicators. These were sometimes called "circuit-component" lamps, the other variety being indicators. A variant of the NE-2 type lamp, the NE-77, had three parallel wires (in a plane) instead of the usual two. It was also intended primarily to be a circuit component.
Small neon lamps are used as indicators in electronic equipment. Called "tuneons" in 1930s radio sets, they were fitted as tuning indicators, and would give a brighter glow as the station was tuned in correctly. Larger lamps are used in neon signage. Neon lamps, due to their low current consumption, are used as nightlights. Because of their comparatively fast response time, in the early development of television neon lamps were used as the light source in many mechanical-scan TV displays. They were also used for a variety of other purposes; since a neon lamp can act as a relaxation oscillator with an added resistor and capacitor, it can be used as a simple flashing lamp or audio oscillator. (See Pearson-Anson effect.)
Neon lamps with several shaped electrodes were used as alphanumerical displays known as Nixie tubes. These have since been replaced by other display devices such as light emitting diodes, vacuum fluorescent displays, and liquid crystal displays. Novelty glow lamps with shaped electrodes (such as flowers and leaves), often coated with phosphors, have been made for artistic purposes. In some of these, the glow that surrounds an electrode is part of the design.
 Unlit and lit neon lamps (NE-2 type) and their light spectrum.
Unlit and lit neon lamps (NE-2 type) and their light spectrum.
In AC-excited lamps, both electrodes produce light, but in a DC-excited lamp, only the negative electrode glows. Thus a neon lamp can be used to distinguish between AC and DC sources and to ascertain the polarity of DC sources.
Colour
Neon indicator lamps are normally orange, and are frequently used with a coloured filter over them to improve contrast and change their colour to red or a redder orange, or less often green.
They can also be filled with argon, krypton, or xenon rather than neon, or mixed with it. While the electrical operating characteristics remain similar, the lamps light with a bluish glow (including some ultraviolet) rather than neon's characteristic reddish-orange glow. Ultraviolet radiation then can be used to excite a phosphor coating inside of the bulb and provide a wide range of various colors, including white.[15] A mixture of neon and krypton can be used for green glow, but nevertheless "green neon" lamps are more commonly phosphor-based.
See also
- Timeline of lighting technology
- 1910 in science
- List of light sources
- Neon lighting
- Neon sign
- Nixie tube
References
- ^ Weeks, Mary Elvira (2003). Discovery of the Elements: Third Edition (reprint). Kessinger Publishing. p. 287. http://books.google.com/books?id=SJIk9BPdNWcC&pg=PA287.
- ^ US 1125476, Georges Claude, "Systems of Illuminating by Luminescent Tubes", issued 1915-01-19]
- ^ "Claude Neon Lights Wins Injunction Suit: Also Gets Rights to Recover Profits and Damages Resulting From Patent Infringement". The New York Times. November 28, 1928. Paid access.
- ^ US patent 1316967, Daniel McFarlan Moore, "Gaseous Conduction Lamp", issued 1919-09-23, assigned to General Electric Company
- ^ "Lamp Inventors 1880-1940: Moore Lamp". The Smithsonian Institution. http://americanhistory.si.edu/lighting/bios/moore.htm.
- ^ Thielen, Marcus (2006-02-10). "LED or Neon". http://www.signweb.com/index.php/channel/12/id/138/. Retrieved 2008-12-30.
- ^ J.W. Tuttle and C.R. Dougherty, ed.s, General Electric Glow Lamp Manual (East Cleveland, Ohio: General Electric Co., 1963). See especially the section "Logic and Computer Applications of Glow Lamps," which presents circuits using neon lamps in "and" gates, "or" gates, inverters, pulse generators, flip-flops, and ring counters. This section is available on-line at: http://computer-refuge.org/classiccmp/neon_lamp_logic/ . Click on "lamp.zip". The second (1966) edition of this booklet is available on-line here: http://www.tech-systems-labs.com/books/GE-lamps.pdf .
- ^ William G. Miller, Using and Understanding Miniature Neon Lamps (Indianapolis, Indiana: Howard W. Sams, 1969).
- ^ A.A. Vuylsteke, "Neon lamp flip-flop and binary counter," Electronics, vol. 26, page 248 (April 1953).
- ^ M.S. Raphael and A.S. Robinson, "Digital storage using neon tubes," Electronics, vol. 29, pages 162-165 (July 1956).
- ^ Charles E. Hendrix, "A study of the neon bulb as a nonlinear circuit element," Proceedings of the Institute of Radio Engineers: Transactions on component parts, vol. 3, no. 2, pages 44-54 (September 1956). (Use of neon lamps in "and" and "or" gates.)
- ^ J.C. Manley and E.F. Buckley, "Neon diode ring counter," Electronics, vol. 23, pages 84-87 (January 1950).
- ^ R.L. Ives, "Neon oscillator rings," Electronics, vol. 31, pages 108-115 (10 October 1958).
- ^ C.E. Hendrix and R.B. Purcell, "Neon lamp logic gates play tic-tac-toe," Electronics, vol. 31, pages 68-69 (20 June 1958).
- ^ Yen, William M.; Yamamoto, Hajime (2007). Phosphor handbook. CRC Press. p. 442. ISBN 9780849335648. http://books.google.com/books?id=I9O1K20-uo4C&pg=PA442.
External links
Lamps and lighting Incandescent Fluorescent High-intensity
discharge (HID)Mercury-vapor · Hydrargyrum medium-arc iodide (HMI) · Hydrargyrum quartz iodide (HQI) · Metal halide (Ceramic) · Sodium vaporGas discharge Deuterium arc · Neon · Sulfur · Xenon arc / Xenon flash · Black light · Tanning lamp · Germicidal · Growth lightElectric arc Combustion Other Lamp (electrical component) · Light fixture · Lightbulb sockets · Light-emitting diode (LED) · LED lamp · Solid-state (SSL) · Plasma · Electroluminescent wire · Chemiluminescence · Radioluminescence · Glow stick · ESLCategories:- Neon lighting
- Vacuum tube displays
- 1902 introductions
- Light sources
Wikimedia Foundation. 2010.


