- Chiton
-
This article is about the mollusc. For the ancient Greek article of dress, see Chiton (costume).
Chiton
Temporal range: Devonian–Recent[1][2]
Lined chiton, Tonicella lineata. The anterior end of the animal is to the right Scientific classification 
Kingdom: Animalia Phylum: Mollusca Class: Polyplacophora
Blainville, 1816Subclasses Chitons (
 /ˈkaɪtənz/) are small to large, primitive marine molluscs in the class Polyplacophora. There are 900 to 1,000 extant species of chitons in the class, which was formerly known as Amphineura.[3]
/ˈkaɪtənz/) are small to large, primitive marine molluscs in the class Polyplacophora. There are 900 to 1,000 extant species of chitons in the class, which was formerly known as Amphineura.[3]These molluscs are also sometimes commonly known as sea cradles or "coat-of-mail shells". They are also sometimes referred to more formally as loricates, polyplacophorans, and rarely as polyplacophores.
Chitons have a dorsal shell which is composed of eight separate shell plates or valves. These plates overlap somewhat at the front and back edges, and yet the plates articulate well with one another. Because of this, although the plates provide good protection for impacts from above, they nonetheless permit the chiton to flex upward when needed for locomotion over uneven surfaces, and also the animal can slowly curl up into a ball when it is dislodged from the underlying surface. The shell plates are surrounded by a structure known as a girdle.
Contents
Habitat
 Two individuals of Acanthopleura granulata on a rock at high tide level in Guadeloupe
Two individuals of Acanthopleura granulata on a rock at high tide level in Guadeloupe
Chitons live worldwide, in cold water and in the tropics. Most of them inhabit intertidal or subtidal zones and do not extend beyond the photic zone.
They live on hard surfaces, such as on or under rocks, or in rock crevices. Some species live quite high in the intertidal zone and are exposed to the air and light for long periods. Others live subtidally. A few species live in deep water, as deep as 6,000 metres (20,000 ft).
Chitons are exclusively and fully marine. This is in contrast to the bivalves which were able to adapt to brackish water and freshwater, and the gastropods which were able to make successful transitions to freshwater and terrestrial environments.
Morphology
Shell
All chitons bear a row of aragonitic shells, although in some species they are reduced or covered by the girdle tissue.[4] The calcareous valves that chitons carry dorsally are protective, made wholly of aragonite,[5] and variously colored, patterned, smooth or sculptured. The shell is divided into eight articulating calcareous (aragonite) valves embedded in the tough muscular girdle that surrounds the chiton's body. This arrangement allows chitons to roll into a protective ball when dislodged and to cling tightly to irregular surfaces.
Part of a series on Seashells 
Mollusk shells About mollusk shells - Conchology
- Nacre
- Valve
Other seashells  Loose valves or plates of Chiton tuberculatus from the beachdrift on Nevis, West Indies. Head plates at the top, tail plates at the bottom
Loose valves or plates of Chiton tuberculatus from the beachdrift on Nevis, West Indies. Head plates at the top, tail plates at the bottom
The most anterior plate is crescent shaped, and is known as the cephalic plate (sometimes called a "head plate", despite the absence of a complete head). The most posterior plate is known as the anal plate (sometimes called the "tail plate", although chitons do not have a tail.)
The front seven plates develop simultaneously, with the rear plate being added later in the developmental process. Growth lines are formed each winter.[6] The inner layer of each of the six intermediate plates is produced anteriorly as an articulating flange. This is called the articulamentum. This inner layer may also be produced laterally in the form of notched insertion plates. These function as an attachment of the valve plates to the soft body. A similar series of insertion plates may be attached to the convex anterior border of the cephalic plate or the convex posterior border of the anal plate.[7]
The sculpture of the valves is one of the taxonomic characteristics, along with the granulation or spinulation of the girdle[7]
After a chiton dies, the individual valves which make up the 8-part shell come apart because the girdle is no longer holding them together, and then the plates sometimes wash up in beach drift. The individual shell plates from a chiton are sometimes known as "butterfly shells" because of their shape.
Girdle ornament
The girdle may be ornamented with scales or spicules which, like the shell plates, are mineralized with aragonite – although a different mineralization process operates in the spicules to in the teeth or shells (implying an independent evolutionary innovation).[5] This process seems quite simple in comparison to other shelly tissue; the crystal structure of the deposited minerals closely resembles the disordered nature of crystals that form inorganically.[5]
The protein component of the scales and sclerites is minuscule in comparison with other biomineralized structures, whereas the total proportion of matrix is higher than in mollusc shells. This implies that polysaccharides make up the bulk of the matrix.[5] The girdle spines often bear length-parallel striations.[8]
The wide form of girdle ornament suggests that it serves a secondary role; chitons can survive perfectly well without them. Camouflage or defence are two likely functions.[5]
Internal anatomy
The girdle is often ornamented with spicules, bristles, hairy tufts, spikes, or snake-like scales. The majority of the body is a snail-like foot, but no head or other soft-parts beyond the girdle are visible from the dorsal side. The mantle cavity consists of a narrow channel on each side, lying between the body and the girdle. Water enters the cavity through openings either side of the mouth, then flows along the channel to a second, exhalant, opening close to the anus.[9] Multiple gills hang down into the mantle cavity along part or all of the lateral pallial groove, each consisting of a central axis with a number of flattened filaments through which oxygen can be absorbed.[10]
The heart has three chambers and is located towards the animal's hind end. Each of the two auricles collects blood from the gills on one side, while the muscular ventricle pumps blood through the aorta and round the body.
The excretory system consists of two nephridia, which connect to the pericardial cavity around the heart, and remove excreta through a pore that opens near the rear of the mantle cavity. The single gonad is located in front of the heart, and releases gametes through a pair of pores just in front of those used for excretion.[10]
 The underside of the gumboot chiton, Cryptochiton stelleri, showing the foot in the center, surrounded by the gills and mantle. The mouth is visible to the left in this image.
The underside of the gumboot chiton, Cryptochiton stelleri, showing the foot in the center, surrounded by the gills and mantle. The mouth is visible to the left in this image.
The mouth is located on the underside of the animal, and contains a tongue-like structure called a radula, which has numerous rows of 17 teeth each. The teeth are coated with magnetite, a hard ferric/ferrous oxide mineral. The radula is used to scrape microscopic algae off the substratum. The mouth cavity itself is lined with chitin and is associated with a pair of salivary glands. Two sacs open from the back of the mouth, one containing the radula, and the other containing a protrusible sensory subradula organ that is pressed against the substratum to taste for food.[10]
Cilia pull the food through the mouth in a stream of mucus and through the oesophagus, where it is partially digested by enzymes from a pair of large pharyngeal glands. The oesophagus in turn opens into a stomach with where enzymes from a digestive gland complete the breakdown of the food. Nutrients are absorbed through the linings of the stomach and the first part of the intestine. The intestine is divided in two by a sphincter, with the latter part being highly coiled and functioning to compact the waste matter into faecal pellets. The anus opens just behind the foot.[10]
Chitons lack a clearly demarcated head; their nervous system resembles a dispersed ladder.[2] There are no true ganglia as there are in other molluscs, although there is a ring of dense neural tissue around the oesophagus. From this ring, nerves branch forwards to innervate the mouth and subradula, while two pairs of main nerve cords run back through the body. One pair, the pedal cords, innervate the foot, while the pallio-visceral cords innervate the mantle and remaining internal organs.[10]
Some species bear an array of tentacles in front of the head.[11]
Senses
Further information: Aesthete (chiton)The primary sense organs of chitons are the subradula organ and a large number of unique organs called aesthetes. The aesthetes consist of light sensitive cells just below the surface of the shell, although they are not capable of true vision. In some cases, however, they are modified to form ocelli, with a cluster of individual photoreceptor cells lying beneath a small lens. An individual chiton may have thousands of such ocelli[10]
There is a relatively good fossil record of chiton shells, but ocelli are only present in those dating to 10 million years ago or younger; this would make the ocelli, whose precise function is unclear, the most recent eyes to evolve.[2]
Although chitons lack osphradia, statocysts, and other sensory organs common to other molluscs, they do have numerous tactile nerve endings, especially on the girdle and within the mantle cavity.
However, chitons lack a cerebral ganglion.[12]
Homing Ability
Several species of Chiton are known to exhibit homing behaviours, journeying to feed and then returning to the exact unique spot they previously inhabited.[13] The specific method of how chitons can perform such behaviors has been investigated to some extent, however the reason remains unknown. Suggestions include the chitons remembering the topographic profile of the region, thus being able to guide themselves back to their home scar via a physical knowledge of the rocks and a visual input by their primitive eyes,[14] The gastropod Nerita textilis is known to deposit mucus as it moves, which a chemoreceptive organ is able to detect and guide the snail back to its home site,[15] It is unclear if chitons function in this manner. However, it is theorized that they may leave chemical cues along the rock surface and at the home scar which olfactory senses can detect and home in on. Furthermore, old trails may be detected, providing further stimulus for the Chiton to find its home,[14] Also, chitons have teeth made of magnetite on their radula making them unique among animals. This means they have an exceptionally abrasive tongue with which to scrape food from rocks. These crystals are thought to be involved in magnetoreception, the ability to sense the polarity or the inclination of the Earth's magnetic field, and to be involved in navigation.
Culinary uses
Chitons are eaten in many islands in the Caribbean, including Trinidad, Tobago and Barbados. They were also eaten by native Americans of the Pacific coasts of both North and South America. The foot of the chiton is prepared in a manner similar to abalone.
Life habits
A chiton creeps along slowly on a muscular foot. They have considerable power of adhesion and can cling to rocks very powerfully, like a limpet.
Chitons are herbivorous grazers. They eat algae, bryozoans, diatoms and sometimes bacteria by scraping the rocky substrate with their well-developed radula.
A few species of chitons are predatory, such as the small western Pacific species Placiphorella velata. These predatory chitons have an enlarged anterior girdle. They catch other small invertebrates, such as shrimp and possibly even small fish, by holding the enlarged, hood-like front end of the girdle up off the surface, and then clamping down on unsuspecting, shelter-seeking prey.
Some chitons exhibit homing behavior, returning to the same spot for the daylight hours and roaming around at night to feed.
Reproduction and life cycle
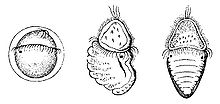 Larvae of chitons: First image is the trochophore, second is in metamorphosis, third is an immature adult.
Larvae of chitons: First image is the trochophore, second is in metamorphosis, third is an immature adult.
Chitons have separate sexes, and fertilisation is external. The male releases sperm into the water, while the female releases eggs either individually, or in a long string. In most cases, fertilisation takes place either in the surrounding water, or in the mantle cavity of the female. Some species brood the eggs within the mantle cavity, and the species Callistochiton viviparus even retains them within the ovary and gives birth to live young, an example of ovoviviparity.
The eggs have a tough spiny coat, and usually hatch to release a free-swimming trochophore larva, typical of many other mollusc groups. In a few cases, the trochophore remains within the egg (and is then called lecithotrophic – deriving nutrition from yolk), which hatches to produce a miniature adult. Unlike most other molluscs, there is no intermediate stage, or veliger, between the trochophore and the adult. Instead, a segmented shell gland forms on one side of the larva, and a foot forms on the opposite side. When the larva is ready to become an adult, the body elongates, and the shell gland secretes the plates of the shell. Unlike the fully grown adult, the larva has a pair of simple eyes, although these may remain for some time in the immature adult.[10]
Predators
Animals which prey on chitons include humans, seagulls, seastars, crabs, lobster and fish.
The largest species
The largest chiton (up to 33 cm in length) is the brick-red gumboot chiton of the Pacific Northwest. In this species the valves are completely internal.
Evolutionary origins
Chitons have a relatively good fossil record, stretching back 400 million years[2] to the Devonian. Before this, some organisms have been interpreted (tentatively) as stem-group polyplacophora; the record of polyplacophora stretches back to the Ordovician.[16]
Separate plates from Matthevia, a Late Cambrian polyplacophoran from the Hellnmaria Member of the Notch Peak Limestone, Steamboat Pass, southern House Range, Utah. The US one cent coin is 19 mm in diameter
Kimberella and Wiwaxia of the Precambrian and Cambrian may be related to ancestral polyplacophora. Matthevia is a Late Cambrian polyplacophoran preserved as individual pointed valves, and sometimes considered to be a chiton,[1] although it can at best be a stem-group member of the group.[17] Based on this and co-occurring fossils, one plausible hypothesis for the origin of polyplacophora has that they formed when an aberrant monoplacophoran was born with multiple centres of calcification, rather than the usual one. Selection quickly acted on the resultant conical shells to form them to overlap into protective armour; their original cones are homologous to the tips of the plates of modern chitons.[1]
The chitons evolved from multiplacophora during the palaeozoic, with their relatively conserved modern-day body plan being fixed by the mesozoic.[17]
History of the scientific investigation of chitons
Chitons were first studied by Carolus Linnaeus in 1758. Since his description of the first four species, chitons have been variously classified. They were called Cyclobranchians ("round arm") in the early 19th century, and then grouped with the aplacophorans in the subphylum Amphineura in 1876. The class Polyplacophora was named by J. E. Gray in 1821.
Etymology
The English name "chiton" originates from the Latin word chitōn, which means "mollusc", and in turn is derived from the Greek word "khitōn", meaning tunic (which also is the source of the word chitin). The Greek word "khitōn" can be traced to the Central Semitic word "*kittan", which is from the Akkadian words "kitû" or "kita’um", meaning flax or linen, and originally the Sumerian word "gada" or "gida".[citation needed]
The Greek-derived name Polyplacophora comes from the words poly- (many), plako- (tablet), and -phoros (bearing), a reference to the chiton's eight shell plates.
Taxonomy
Most classification schemes in use today are based, at least in part, on Pilsbry's Manual of Conchology (1892–1894), extended and revised by Kaas and Van Belle (1985–1990).
Since chitons were first described by Linnaeus (1758) there have been extensive taxonomic studies at the species level. However, the taxonomic classification at higher levels in the group has remained somewhat unsettled.
The most recent classification (Sirenko 2006) is based not only on shell morphology, as usual, but also other important features including aesthetes, girdle, radula, gills, glands, egg hull projections and spermatozoids. It includes all the living and extinct genera of chitons.
This system is now generally accepted.
- Class Polyplacophora Gray, 1821
- Subclass Paleoloricata Bergenhayn, 1955
- Order Chelodida Bergenhayn, 1943
-
- Family Chelodidae Bergenhayn, 1943
- Chelodes Davidson et King, 1874
- Euchelodes Marek, 1962
- Calceochiton Flower, 1968
- Family Chelodidae Bergenhayn, 1943
-
- Order Septemchitonida Bergenhayn, 1955
-
- Family Gotlandochitonidae Bergenhayn, 1955
- Gotlandochiton Bergenhayn, 1955
- Family Helminthochitonidae Van Belle, 1975
- Kindbladochiton Van Belle, 1975
- Diadelochiton Hoare, 2000
- Helminthochiton Salter in Griffith et M'Coy, 1846
- Echinochiton Pojeta, Eernisse, Hoare et Henderson, 2003
- Family Septemchitonidae Bergenhayn, 1955
- Septemchiton Bergenhayn, 1955
- Paleochiton A. G. Smith, 1964
- Thairoplax Cherns, 1998
- Family Gotlandochitonidae Bergenhayn, 1955
-
- Order Chelodida Bergenhayn, 1943
- Subclass Loricata Shumacher, 1817
- Order Lepidopleurida Thiele, 1910
- Suborder Cymatochitonina Sirenko et Starobogatov, 1977
- Family Acutichitonidae Hoare, Mapes et Atwater, 1983
- Acutichiton Hoare, Sturgeon et Hoare, 1972
- Elachychiton Hoare, Sturgeon et Hoare, 1972
- Harpidochiton Hoare et Cook, 2000
- Arcochiton Hoare, Sturgeon et Hoare, 1972
- Kraterochiton Hoare, 2000
- Soleachiton Hoare, Sturgeon et Hoare, 1972
- Asketochiton Hoare et Sabattini, 2000
- Family Cymatochitonidae Sirenko et Starobogatov, 1977
- Cymatochiton Dall, 1882
- Compsochiton Hoare et Cook, 2000
- Family Gryphochitonidae Pilsbry, 1900
- Gryphochiton Gray, 1847
- Family Lekiskochitonidae Smith et Hoare, 1987
- Lekiskochiton Hoare et Smith, 1984
- Family Permochitonidae Sirenko et Starobogatov, 1977
- Permochiton Iredale et Hull, 1926
- Family Acutichitonidae Hoare, Mapes et Atwater, 1983
- Suborder Lepidopleurina Thiele, 1910
- Family Ferreiraellidae Dell’ Angelo et Palazzi, 1991
- Glaphurochiton Raymond, 1910
- ?Pyknochiton Hoare, 2000
- ?Hadrochiton Hoare, 2000
- Ferreiraella Sirenko, 1988
- Family Glyptochitonidae Starobogatov et Sirenko, 1975
- Glyptochiton Konninck, 1883
- Family Leptochitonidae Dall, 1889
- Colapterochiton Hoare et Mapes, 1985
- Coryssochiton DeBrock, Hoare et Mapes, 1984
- Proleptochiton Sirenko et Starobogatov, 1977
- Schematochiton Hoare, 2002
- Pterochiton (Carpenter MS) Dall, 1882
- Leptochiton Gray, 1847
- Parachiton Thiele, 1909
- Terenochiton Iredale, 1914
- Trachypleura Jaeckel, 1900
- Pseudoischnochiton Ashby, 1930
- Lepidopleurus Risso, 1826
- Hanleyella Sirenko, 1973
- Family Camptochitonidae Sirenko, 1997
- Camptochiton DeBrock, Hoare et Mapes, 1984
- Pedanochiton DeBrock, Hoare et Mapes, 1984
- Euleptochiton Hoare et Mapes, 1985
- Pileochiton DeBrock, Hoare et Mapes, 1984
- Chauliochiton Hoare et Smith, 1984
- Stegochiton Hoare et Smith, 1984
- Family Nierstraszellidae Sirenko, 1992
- Nierstraszella Sirenko, 1992
- Family Mesochitonidae Dell’ Angelo et Palazzi, 1989
- Mesochiton Van Belle, 1975
- Pterygochiton Rochebrune, 1883
- Family Protochitonidae Ashby, 1925
- Family Hanleyidae Bergenhayn, 1955
- Hanleya Gray, 1857
- Hemiarthrum Dall, 1876
- Family Ferreiraellidae Dell’ Angelo et Palazzi, 1991
- Suborder Cymatochitonina Sirenko et Starobogatov, 1977
- Order Chitonida Thiele, 1910
- Suborder Chitonina Thiele, 1910
- Superfamily Chitonoidea Rafinesque, 1815
- Family Ochmazochitonidae Hoare et Smith, 1984
- Ochmazochiton Hoare et Smith, 1984
- Family Ischnochitonidae Dall, 1889
- Ischnochiton Gray, 1847
- Stenochiton H. Adams et Angas, 1864
- Stenoplax (Carpenter MS) Dall, 1879
- Lepidozona Pilsbry, 1892
- Stenosemus Middendorff, 1847
- Subterenochiton Iredale et Hull, 1924
- Thermochiton Saito et Okutani, 1990
- Connexochiton Kaas, 1979
- Tonicina Thiele, 1906
- Family Callistoplacidae Pilsbry, 1893
- Family Chaetopleuridae Plate, 1899
- Chaetopleura Shuttleworth, 1853
- Dinoplax Carpenter MS, Dall, 1882[18]
- Family Loricidae Iredale et Hull, 1923
- Family Callochitonidae Plate, 1901
- Callochiton Gray, 1847
- Eudoxochiton Shuttleworth, 1853
- Vermichiton Kaas, 1979
- Family Chitonidae Rafinesque, 1815
- Subfamily Chitoninae Rafinesque, 1815
- Chiton Linnaeus, 1758
- Amaurochiton Thiele, 1893
- Radsia Gray, 1847
- Sypharochiton Thiele, 1893
- Nodiplax Beu, 1967
- Rhyssoplax Thiele, 1893
- Teguloaplax Iredale & Hull, 1926
- Mucrosquama Iredale, 1893
- Subfamily Toniciinae Pilsbry, 1893
- Tonicia Gray, 1847
- Onithochiton Gray, 1847
- Subfamily Acanthopleurinae Dall, 1889
- Acanthopleura Guilding, 1829
- Liolophura Pilsbry, 1893
- Enoplochiton Gray, 1847
- Squamopleura Nierstrasz, 1905
- Superfamily Schizochitonoidea Dall, 1889
- Family Schizochitonidae Dall, 1889
- Incissiochiton Van Belle, 1985
- Schizochiton Gray, 1847
- Family Ochmazochitonidae Hoare et Smith, 1984
- Suborder Acanthochitonina Bergenhayn, 1930
- Superfamily Mopalioidea Dall, 1889
- Family Tonicellidae Simroth, 1894
- Subfamily Tonicellinae Simroth, 1894
- Subfamily Juvenichitoninae Sirenko, 1975
- Juvenichiton Sirenko, 1975
- Micichiton Sirenko, 1975
- Nanichiton Sirenko, 1975
- Family Schizoplacidae Bergenhayn, 1955
- Schizoplax Dall, 1878
- Family Mopaliidae Dall, 1889
- Subfamily Heterochitoninae Van Belle, 1978
- Heterochiton Fucini, 1912
- Allochiton Fucini, 1912
- Subfamily Mopaliinae Dall, 1889
- Aerilamma Hull, 1924
- Guildingia Pilsbry, 1893
- Frembleya H. Adams, 1866
- Diaphoroplax Iredale, 1914
- Plaxiphora Gray, 1847
- Placiphorina Kaas & Van Belle, 1994
- Nuttallochiton Plate, 1899
- Mopalia Gray, 1847
- Maorichiton Iredale, 1914
- Placiphorella (Carpenter MS) Dall, 1879
- Katharina Gray, 1847
- Amicula Gray, 1847
- Superfamily Cryptoplacoidea H. et A. Adams, 1858
- Family Acanthochitonidae Pilsbry, 1893
- Subfamily Acanthochitoninae Pilsbry, 1893
- Acanthochitona Gray, 1921
- Craspedochiton Shuttleworth, 1853
- Spongiochiton (Carpenter MS) Dall, 1882
- Notoplax H. Adams, 1861
- Pseudotonicia Ashby, 1928
- Bassethullia Pilsbry, 1928
- Americhiton Watters, 1990
- Choneplax (Carpenter MS) Dall, 1882
- Cryptoconchus (de Blainville MS) Burrow, 1815
- Subfamily Cryptochitoninae Pilsbry, 1893
- Cryptochiton Middendorff, 1847
- Family Hemiarthridae Sirenko, 1997
- Hemiarthrum Carpenter in Dall, 1876
- Weedingia Kaas, 1988
- Family Choriplacidae Ashby, 1928
- Choriplax Pilsbry, 1894
- Family Cryptoplacidae H. et A. Adams, 1858
- Cryptoplax de Blainville, 1818
- Order Lepidopleurida Thiele, 1910
- Incertae sedis
-
-
- Family Scanochitonidae Bergenhayn, 1955
- Scanochiton Bergenhayn, 1955
- Family Olingechitonidae Starobogatov et Sirenko, 1977
- Olingechiton Bergenhayn, 1943
- Family Haeggochitonidae Sirenko et Starobogatov, 1977
- Haeggochiton Bergenhayn, 1955
- Family Ivoechitonidae Sirenko et Starobogatov, 1977
- Ivoechiton Bergenhayn, 1955
- Family Scanochitonidae Bergenhayn, 1955
-
-
- Subclass Paleoloricata Bergenhayn, 1955
References
- ^ a b c Runnegar, B.; Pojeta J, J. (Oct 1974). "Molluscan Phylogeny: the Paleontological Viewpoint". Science 186 (4161): 311–317. Bibcode 1974Sci...186..311R. doi:10.1126/science.186.4161.311. JSTOR 1739764. PMID 17839855.
- ^ a b c d Serb, J. M.; Eernisse, D. J. (2008). "Charting Evolution’s Trajectory: Using Molluscan Eye Diversity to Understand Parallel and Convergent Evolution". Evolution Education and Outreach 1 (4): 439–447. doi:10.1007/s12052-008-0084-1.
- ^ "Polyplacophora". Integrated Taxonomic Information System. http://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=78807.
- ^ Vinther, J.; Nielsen, C. (2005). "The Early Cambrian Halkieria is a mollusc". Zoologica Scripta 34: 81. doi:10.1111/j.1463-6409.2005.00177.x.
- ^ a b c d e Treves, K.; Traub, W.; Weiner, S.; Addadi, L. (2003). "Aragonite Formation in the Chiton (Mollusca) Girdle". Helvetica Chimica Acta 86 (4): 1101. doi:10.1002/hlca.200390096.
- ^ Boolootian, R. A. (1964). "On growth, feeding and reproduction in the chitonMopalia muscosa of Santa Monica Bay". Helgoländer Wissenschaftliche Meeresuntersuchungen 11 (3–4): 186–199. Bibcode 1964HWM....11..186B. doi:10.1007/BF01612371.
- ^ a b P.J. Hayward, and J.S. Ryland (1996). Handbook of the Marine Fauna of North-West Europe. Oxford University Press. pp. 485. ISBN 0198540558.
- ^ Treves, K.; Traub, W.; Weiner, S.; Addadi, L. (2003). "Aragonite Formation in the Chiton (Mollusca) Girdle". Helvetica Chimica Acta 86 (4): 1101. doi:10.1002/hlca.200390096.
- ^ animalnetwork.com
- ^ a b c d e f g Barnes, Robert D. (1982). Invertebrate Zoology. Philadelphia, PA: Holt-Saunders International. pp. 381–389. ISBN 0-03-056747-5.
- ^ "FEEDING BEHAVIOR OF THE CHITON PLACIPHORELLA". Journal of Molluscan Studies 35 (1): 23. 1962-04-01. http://mollus.oxfordjournals.org/content/35/1/23.full.
- ^ (Thorne. J. M, 1968; Moroz. L, et al, 1993).
- ^ Chelazzi, et al, 1983, 'A comparative study on the movement pattern of two sympatric tropical chitons, Mollusca: Polyplacophora', Marine Biology, vol 74, pp 115-125.; Chelazzi. G, et al, 1990, 'The role of trail folliwing in the homing of intertidal chitons: a comparison between three Acanthopleura spp', Marine Biology, vol 105, pp 445-450.
- ^ a b (Chelazzi. G, et al, 1987; Thorne. J M, 1968).
- ^ (Chelazzi. G, et al, 1985).
- ^ Sigwart; Sutton, M. D. (Oct 2007). "Deep molluscan phylogeny: synthesis of palaeontological and neontological data". Proceedings of the Royal Society B: Biological sciences 274 (1624): 2413–2419. doi:10.1098/rspb.2007.0701. PMC 2274978. PMID 17652065. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2274978. For a summary, see "The Mollusca". University of California Museum of Paleontology. http://www.ucmp.berkeley.edu/taxa/inverts/mollusca/mollusca.php. Retrieved 2008-10-02.
- ^ a b Vendrasco, M. J.; Wood, T. E.; Runnegar, B. N. (2004). "Articulated Palaeozoic fossil with 17 plates greatly expands disparity of early chitons". Nature 429 (6989): 288–291. Bibcode 2004Natur.429..288V. doi:10.1038/nature02548. PMID 15152250.
- ^ http://www.marinespecies.org/aphia.php search term Dinoplax accessed 7 April 2010
- Sirenko BI. New outlook on the system of chitons (Mollusca: Polyplacophora). Venus, 65 (1-2): 27-49, 2006
External links
Categories:- Chitons
Wikimedia Foundation. 2010.


