- Vanillin
-
Vanillin 
 4-Hydroxy-3-methoxybenzaldehyde[1]Other names
4-Hydroxy-3-methoxybenzaldehyde[1]Other namesIdentifiers CAS number 121-33-5 
PubChem 1183 ChemSpider 13860434 
UNII CHI530446X 
EC number 204-465-2 KEGG D00091 
MeSH vanillin ChEBI CHEBI:18346 
ChEMBL CHEMBL13883 
RTECS number YW5775000 Beilstein Reference 472792 Gmelin Reference 3596 3DMet B00167 Jmol-3D images Image 1 - COC1C=C(C=O)C=CC1=O
Properties Molecular formula C8H8O3 Molar mass 152.15 g mol−1 Exact mass 152.047344122 g mol-1 Appearance White crystals Odor Floral, pleasant Density 1.056 g cm-3 Melting point 81-83 °C, 354-356 K, 178-181 °F
Boiling point 285 °C, 558 K, 545 °F
Solubility in water 10 g dm-3 log P 1.208 Vapor pressure >1 Pa Acidity (pKa) 7.781 Basicity (pKb) 6.216 Structure Crystal structure Monoclinic Hazards MSDS hazard.com GHS pictograms 
GHS signal word WARNING GHS hazard statements H302, H317, H319 GHS precautionary statements P280, P305+351+338 EU classification  Xn
XnR-phrases R22 NFPA 704 Flash point 147 °C Related compounds Related compounds Anisaldehyde
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Vanillin is a phenolic aldehyde, an organic compound with the molecular formula C8H8O3. Its functional groups include aldehyde, ether, and phenol. It is the primary component of the extract of the vanilla bean. It is also found in Leptotes bicolor,[3] roasted coffee[4] and the Chinese red pine. Synthetic vanillin, instead of natural vanilla extract, is sometimes used as a flavoring agent in foods, beverages, and pharmaceuticals.
Vanillin as well as ethylvanillin is used by the food industry. The ethyl is more expensive but has a stronger note. It differs from vanillin by having an ethoxy group (–O–CH2CH3) instead of a methoxy group (–O–CH3).
Natural "vanilla extract" is a mixture of several hundred different compounds in addition to vanillin. Artificial vanilla flavoring is a solution of pure vanillin, usually of synthetic origin. Because of the scarcity and expense of natural vanilla extract, there has long been interest in the synthetic preparation of its predominant component. The first commercial synthesis of vanillin began with the more readily available natural compound eugenol. Today, artificial vanillin is made from either guaiacol or from lignin, a constituent of wood which is a byproduct of the pulp industry.
Lignin-based artificial vanilla flavoring is alleged to have a richer flavor profile than oil-based flavoring; the difference is due to the presence of acetovanillone in the lignin-derived product, an impurity not found in vanillin synthesized from guaiacol.[5]
Contents
History
Vanilla was cultivated as a flavoring by pre-Columbian Mesoamerican peoples; at the time of their conquest by Hernán Cortés, the Aztecs used it as a flavoring for chocolate. Europeans became aware of both chocolate and vanilla around 1520.[6]
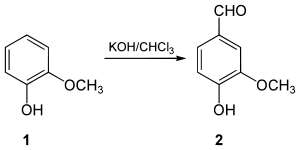
Vanillin was first isolated as a relatively pure substance in 1858 by Nicolas-Theodore Gobley, who obtained it by evaporating a vanilla extract to dryness, and recrystallizing the resulting solids from hot water.[7] In 1874, the German scientists Ferdinand Tiemann and Wilhelm Haarmann deduced its chemical structure, at the same time finding a synthesis for vanillin from coniferin, a glycoside of isoeugenol found in pine bark.[8] Tiemann and Haarmann founded a company, Haarmann & Reimer (now part of Symrise) and started the first industrial production of Vanillin using their process in Holzminden (Germany). In 1876, Karl Reimer synthesized vanillin (2) from guaiacol (1).[9]
By the late 19th century, semisynthetic vanillin derived from the eugenol found in clove oil was commercially available.[10]
Synthetic vanillin became significantly more available in the 1930s, when production from clove oil was supplanted by production from the lignin-containing waste produced by the Sulfite pulping process for preparing wood pulp for the paper industry. By 1981, a single pulp and paper mill in Ontario supplied 60% of the world market for synthetic vanillin.[11] However, subsequent developments in the wood pulp industry have made its lignin wastes less attractive as a raw material for vanillin synthesis. While some vanillin is still made from lignin wastes, most synthetic vanillin is today synthesized in a two-step process from the petrochemical precursors guaiacol and glyoxylic acid.[6]
Beginning in 2000, Rhodia began marketing biosynthetic vanillin prepared by the action of microorganisms on ferulic acid extracted from rice bran. At $700/kg, this product, sold under the trademarked name Rhovanil Natural, is not cost-competitive with petrochemical vanillin, which sells for around $15/kg.[12] However, unlike vanillin synthesized from lignin or guaiacol, it can be labeled as a natural flavoring.
Occurrence
Vanillin is most prominent as the principal flavor and aroma compound in vanilla. Cured vanilla pods contain approximately 2% by dry weight vanillin; on cured pods of high quality, relatively pure vanillin may be visible as a white dust or "frost" on the exterior of the pod.
At lower concentrations, vanillin contributes to the flavor and aroma profiles of foodstuffs as diverse as olive oil,[13] butter,[14] raspberry[15] and lychee[16] fruits. Aging in oak (wine) barrels imparts vanillin to some wines and spirits.[17] In other foods, heat treatment evolves vanillin from other chemicals. In this way, vanillin contributes to the flavor and aroma of coffee,[18] maple syrup,[19] and whole grain products including corn tortillas[20] and oatmeal.[21]
Production
Natural production
Natural vanillin is extracted from the seed pods of Vanilla planifola, a vining orchid native to Mexico, but now grown in tropical areas around the globe. Madagascar is presently the largest producer of natural vanillin.
As harvested, the green seed pods contain vanillin in the form of its β-D-glycoside; the green pods do not have the flavor or odor of vanilla.[22]
After being harvested, their flavor is developed by a months-long curing process, the details of which vary among vanilla-producing regions, but in broad terms it proceeds as follows:
First, the seed pods are blanched in hot water, to arrest the processes of the living plant tissues. Then, for 1–2 weeks, the pods are alternately sunned and sweated: during the day, they are laid out in the sun, and each night, wrapped in cloth and packed in airtight boxes to sweat. During this process, the pods become a dark brown, and enzymes in the pod release vanillin as the free molecule. Finally, the pods are dried and further aged for several months, during which time their flavors further develop. Several methods have been described for curing vanilla in days rather than months, although they have not been widely developed in the natural vanilla industry,[23] with its focus on producing a premium product by established methods, rather than on innovations that might alter the product's flavor profile.
Vanillin accounts for about 2% of the dry weight of cured vanilla beans, and is the chief among about 200 other flavor compounds found in vanilla.
Biosynthesis
The biosynthesis of vanillin is achieved by the conversion of tyrosine into 4-coumaric acid then into ferulic acid and finally into vanillin. Vanillin is then converted into its corresponding glucose ester.
 Biosynthesis of Vanillin From Tyrosine
Biosynthesis of Vanillin From Tyrosine
The conversion of ferulic acid into vanillin is achieved by conversion of the carboxylic acid into a thioester with acetyl-CoA. The feruloyl CoA is then hydrated into 4-hydroxy-3-methoxyphenyl-β-hydroxyprpionyl CoA (HMPHP CoA). At this point, two different pathways have been purposed for the conversion of HMPHP CoA into vanillin. One pathway is similar to the β-oxidation of fatty acid, beginning with the oxidation of the hydroxyl group, cleavage to release acetyl-CoA to form a shortened thioester and then cleavage of the thioester into an aldehyde. The other pathway contains one enzyme that would simultaneously oxidize the hydroxyl group along with the release of aceyl-CoA.
Chemical synthesis
The demand for vanilla flavoring has long exceeded the supply of vanilla beans. As of 2001[update], the annual demand for vanillin was 12,000 tons, but only 1,800 tons of natural vanillin were produced.[24] The remainder was produced by chemical synthesis. Vanillin was first synthesized from eugenol (found in oil of clove) in 1874–75, less than 20 years after it was first identified and isolated. Vanillin was commercially produced from eugenol until the 1920s.[25] Later it was synthesized from lignin-containing "brown liquor", a byproduct of the sulfite process for making wood pulp.[11] Counter-intuitively, even though it uses waste materials, the lignin process is no longer popular because of environmental concerns, and today most vanillin is produced from the petrochemical raw material guaiacol.[11] Several routes exist for synthesizing vanillin from guaiacol.[26]
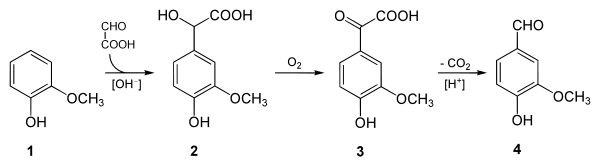
At present, the most significant of these is the two-step process practiced by Rhodia since the 1970s, in which guaiacol (1) reacts with glyoxylic acid by electrophilic aromatic substitution. The resulting vanillylmandelic acid (2) is then converted via 4-Hydroxy-3-methoxyphenylglyoxylic acid (3) to vanillin (4) by oxidative decarboxylation.[6]
In October 2007 Mayu Yamamoto of the International Medical Center of Japan won an Ig Nobel Prize for developing a way to extract vanillin from cow dung.[27]
Uses
The largest use of vanillin is as a flavoring, usually in sweet foods. The ice cream and chocolate industries together comprise 75% of the market for vanillin as a flavoring, with smaller amounts being used in confections and baked goods.[28]
Vanillin is also used in the fragrance industry, in perfumes, and to mask unpleasant odors or tastes in medicines, livestock fodder, and cleaning products.[6] It is also used in the flavor industry, as a very important key note for many different flavors, specially creamy profiles.
Vanillin has been used as a chemical intermediate in the production of pharmaceuticals and other fine chemicals. In 1970, more than half the world's vanillin production was used in the synthesis of other chemicals,[11] but as of 2004 this use accounts for only 13% of the market for vanillin.[29]
Additionally, vanillin can be used as a general purpose stain for developing thin layer chromatography (TLC) plates to aid in visualizing components of a reaction mixture. This stain yields a range of colors for these different components.
Adverse effects
Vanillin can trigger migraine headaches in a small fraction of the people who experience migraines.[30]
See also
References
- Adahchour, Mohamed; René J. J. Vreuls, Arnold van der Heijden and Udo A. Th. Brinkman (1999). "Trace-level determination of polar flavour compounds in butter by solid-phase extraction and gas chromatography–mass spectrometry". Journal of Chromatography A 844 (1–2): 295–305. doi:10.1016/S0021-9673(99)00351-9. PMID 10399332.
- Blank, Imre; Alina Sen, and Werner Grosch (1992). "Potent odorants of the roasted powder and brew of Arabica coffee". Zeitschrift für Lebensmittel-Untersuchung und -Forschung A 195 (3): 239–245. doi:10.1007/BF01202802.
- Brenes, Manuel; Aranzazu García, Pedro García, José J. Rios, and Antonio Garrido (1999). "Phenolic Compounds in Spanish Olive Oils". Journal of Agricultural and Food Chemistry 47 (9): 3535–3540. doi:10.1021/jf990009o. PMID 10552681.
- Buttery, Ron G.; and Louisa C. Ling (1995). "Volatile Flavor Components of Corn Tortillas and Related Products". Journal of Agricultural and Food Chemistry 43 (7): 1878–1882. doi:10.1021/jf00055a023.
- Dignum, Mark J. W.; Josef Kerlera, and Rob Verpoorte (2001). "Vanilla Production: Technological, Chemical, and Biosynthetic Aspects". Food Reviews International 17 (2): 119–120. doi:10.1081/FRI-100000269. http://taylorandfrancis.metapress.com/link.asp?id=db9u44c22f9dqt7w. Retrieved 2006-09-09.
- Esposito, Lawrence J.; K. Formanek, G. Kientz, F. Mauger, V. Maureaux, G. Robert, and F. Truchet (1997). "Vanillin". Kirk-Othmer Encyclopedia of Chemical Technology, 4th edition. 24. New York: John Wiley & Sons. pp. 812–825.
- Fund for Research into Industrial Development, Growth and Equity (FRIDGE) (2004). Study into the Establishment of an Aroma and Fragrance Fine Chemicals Value Chain in South Africa, Part Three: Aroma Chemicals Derived from Petrochemical Feedstocks. National Economic Development and Labor Council. http://www.nedlac.org.za/top.asp?inc=research/fridge/aroma/index.html.
- Gobley, N.-T. (1858). "Recherches sur le principe odorant de la vanille". Journal de Pharmacie et de Chimie 34: 401–405. http://books.google.com/books?id=Yrs8AAAAcAAJ&pg=PA401#v=onepage&q&f=false.
- Guth, Helmut; and Werner Grosch (1995). "Odorants of extrusion products of oat meal: Changes during storage". Zeitschrift für Lebensmittel-Untersuchung und -Forschung A 196 (1): 22–28. doi:10.1007/BF01192979.
- Hocking, Martin B. (September 1997). "Vanillin: Synthetic Flavoring from Spent Sulfite Liquor" (PDF). Journal of Chemical Education 74 (9): 1055–1059. doi:10.1021/ed074p1055. http://jchemed.chem.wisc.edu/hs/Journal/Issues/1997/Sep/abs1055.html. Retrieved 2006-09-09.
- Kermasha, S.; M. Goetghebeur, and J. Dumont (1995). "Determination of Phenolic Compound Profiles in Maple Products by High-Performance Liquid Chromatography". Journal of Agricultural and Food Chemistry 43 (3): 708–716. doi:10.1021/jf00051a028.
- Lampman, Gary M.; Jennifer Andrews, Wayne Bratz, Otto Hanssen, Kenneth Kelley, Dana Perry, and Anthony Ridgeway (1977). "Preparation of vanillin from eugenol and sawdust". Journal of Chemical Education 54 (12): 776–778. doi:10.1021/ed054p776.
- Ong, Peter K. C.; Terry E. Acree (1998). "Gas Chromatography/Olfactory Analysis of Lychee (Litchi chinesis Sonn.)". Journal of Agricultural and Food Chemistry 46 (6): 2282–2286. doi:10.1021/jf9801318.
- Reimer, K. (1876). "Ueber eine neue Bildungsweise aromatischer Aldehyde". Berichte der deutschen chemischen Gesellschaft 9 (1): 423–424. doi:10.1002/cber.187600901134.
- Roberts, Deborah D.; Terry E. Acree (1996). "Effects of Heating and Cream Addition on Fresh Raspberry Aroma Using a Retronasal Aroma Simulator and Gas Chromatography Olfactometry". Journal of Agricultural and Food Chemistry 44 (12): 3919–3925. doi:10.1021/jf950701t.
- Rouhi, A. Maureen (2003). "Fine Chemicals Firms Enable Flavor And Fragrance Industry". Chemical and Engineering News 81 (28): 54.
- Tiemann, Ferd.; Wilh. Haarmann (1874). "Ueber das Coniferin und seine Umwandlung in das aromatische Princip der Vanille". Berichte der Deutschen Chemischen Gesellschaft 7 (1): 608–623. doi:10.1002/cber.187400701193.
- Van Ness, J. H. (1983). "Vanillin". Kirk-Othmer Encyclopedia of Chemical Technology, 3rd edition. 23. New York: John Wiley & Sons. pp. 704–717.
- Viriot, Carole; Augustin Scalbert, Catherine Lapierre, and Michel Moutounet (1993). "Ellagitannins and lignins in aging of spirits in oak barrels". Journal of Agricultural and Food Chemistry 41 (11): 1872–1879. doi:10.1021/jf00035a013.
- Walton, Nicholas J.; Melinda J. Mayer, and Arjan Narbad (July 2003). "Vanillin". Phytochemistry 63 (5): 505–515. doi:10.1016/S0031-9422(03)00149-3.
Notes
- ^ a b PubChem 1183
- ^ Vanillin
- ^ "Leptotes bicolor". Flora Library. http://www.floralibrary.com/flora/leptotes/bicolor. Retrieved 2011-08-21.
- ^ Semmelroch, P.; Laskawy, G.; Blank, I.; Grosch, W. (1995). "Determination of potent odourants in roasted coffee by stable isotope dilution assays". Flavour and Fragrance Journal 10: 1. doi:10.1002/ffj.2730100102.
- ^ According to Esposito 1997, blind taste-testing panels cannot distinguish between the flavors of synthetic vanillin from lignin and from guaicol, but can distinguish the odors of these two types of synthetic vanilla extracts. Guaiacol vanillin, adulterated with acetovanillone, has an odor indistinguishable from lignin vanillin.
- ^ a b c d Esposito 1997
- ^ Gobley 1858
- ^ Tiemann 1874
- ^ Reimer 1876
- ^ According to Hocking 1997, synthetic vanillin was sold commercially in 1874, the same year Tiemann and Haarmann's original synthesis was published. Haarmann & Reimer, one of the corporate ancestors of the modern flavor and aroma manufacturer Symrise, was in fact established in 1874. However, Esposito 1997 claims that synthetic vanillin first became available in 1894 when Rhône-Poulenc (since 1998, Rhodia) entered the vanillin business. If the former claim is correct, the authors of the latter article, being employees of Rhône-Poulenc, may have been unaware of any previous vanillin manufacture.
- ^ a b c d Hocking 1997
- ^ Rouhi 2003
- ^ Brenes 1999
- ^ Adahchour 1999
- ^ Roberts 1996
- ^ Ong 1998
- ^ Viriot 1993
- ^ Blank 1992
- ^ Kermasha 1995
- ^ Buttery 1995
- ^ Guth 1993
- ^ Walton 2003
- ^ Dignum 2001 reviews several such proposed innovations in vanilla processing, including processes in which the seed pods are chopped, frozen, warmed by a heat source other than the sun, or crushed and treated by various enzymes. Whether or not these procedures produce a product whose taste is comparable to traditionally prepared natural vanilla, many of them are incompatible with the customs of the natural vanilla market, in which the vanilla beans are sold whole, and graded by, among other factors, their length.
- ^ Dignum 2001
- ^ Hocking 1997. This chemical process can be conveniently carried out on the laboratory scale using the procedure described by Lampman 1977.
- ^ Van Ness 1983
- ^ Japan’s 12th Ig Noble Prize Winner: Mayu Yamamoto & Dung Vanilla : Japan Probe
- ^ FRIDGE 2004, p. 33
- ^ FRIDGE 2004, p. 32.
- ^ Saint Denis, M.; Coughtrie, MW.; Guilland, JC.; Verges, B.; Lemesle, M.; Giroud, M. (Dec 1996). "[Migraine induced by vanillin].". Presse Med 25 (40): 2043. PMID 9082382.
Categories:- Aldehydes
- Aromatic compounds
- Flavors
- Perfume ingredients
- Phenol ethers
- Vanilla
- Vanilloids
Wikimedia Foundation. 2010.