- Guaiacol
-
Guaiacol[1] 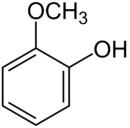
 2-methoxyphenolOther nameso-Methoxyphenol; Methylcatechol[2]
2-methoxyphenolOther nameso-Methoxyphenol; Methylcatechol[2]Identifiers CAS number 90-05-1 
PubChem 460 ChemSpider 447 
UNII 6JKA7MAH9C 
KEGG D00117 
ChEBI CHEBI:28591 
ChEMBL CHEMBL13766 
Jmol-3D images Image 1 - COc1ccccc1O
Properties Molecular formula C7H8O2 Molar mass 124.14 g/mol Density 1.112 g/cm3, liquid
1.129 g/cm3, crystalsMelting point 28 °C, 301 K, 82 °F
Boiling point 204-206 °C
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Guaiacol is a naturally occurring organic compound with the formula C6H4(OH)(OCH3). Although it is biosynthesized by a variety of organisms,[3] this colorless aromatic oil is usually derived from guaiacum or wood creosote. Samples darken upon exposure to air and light. Guaiacol is present in wood smoke, resulting from the pyrolysis of lignin. The compound contributes to the flavor of many compounds, e.g. roasted coffee.[4]
Contents
Preparation
In industry, guaiacol is produced by methylation of catechol, e.g. using potash and dimethyl sulfate:[5]
- C6H4(OH)2 + (CH3O)2SO2 → C6H4(OH)(OCH3) + HO(CH3O)SO2
Laboratory methods
Guaiacol can be prepared by diverse routes in the laboratory. 2-Aminoanisole, derived in two steps from anisole, can be hydrolyzed via its diazonium derivative. Guaiacol can be synthesized by the dimethylation of catechol followed by selective mono-demethylation.[6]
- C6H4(OCH3)2 + C2H5SNa → C6H4(OCH3)(ONa) + C2H5SCH3
Uses
Guaiacol is a precursor to various flavorants such as eugenol[7] and vanillin.[8] Its derivatives are used medicinally as an expectorant, antiseptic, and local anesthetic. It also can be used as a dye in chemical reactions, as oxygen will turn guaiacol from colorless to brown.
Related compounds
Guaiacol carbonate is known as duotal, the phosphate as phosphatol, the phosphite as guaiaco-phosphal (phosphotal is a mixture of the phosphites of creosote phenols).[citation needed] The valerianic ester of guaiacol is known as geosote, the benzoic as benzosol, the salicylic as guaiacolsalol, while the glycerin ether is the drug guaifenesin. The related derivative, dimethoxybenzene or veratrole, is also useful. In preparation of food by smoking, guaiacol is the main chemical responsible for the smoky taste, whereas syringol is responsible for the smoky aroma.
Safety
Methoxyphenols are potential biomarkers of biomass smoke exposure, e.g. from inhalation of woodsmoke. Dietary sources of methoxyphenols overwhelm the contribution from inhalational exposures to woodsmoke.[9]
Locust pheromone
Guaiacol is produced in the gut of locusts, Schistocerca gregaria, by the breakdown of plant material. This process is undertaken by the gut bacterium Pantoea (Enterobacter) agglomerans. Guaiacol is one of the main components of the pheromones that cause locust swarming.[10]
References
- ^ Merck Index, 13th Edition, 4568.
- ^ Chemindustry list of synonyms for guaiacol
- ^ See for example, Duffey, S. S.; Aldrich, J. R.; Blum, M. S. (1977). "Biosynthesis of phenol and guaiacol by the hemipteran Leptoglossus phyllopus". Comparative Biochemistry and Physiology, Part B: Biochemistry & Molecular Biology 56 (2B): 101–102. doi:10.1016/0305-0491(77)90029-3.
- ^ Dorfner, R; Ferge, T; Kettrup, A; Zimmermann, R; Yeretzian, C (Sep 2003). "Real-time monitoring of 4-vinylguaiacol, guaiacol, and phenol during coffee roasting by resonant laser ionization time-of-flight mass spectrometry". Journal of agricultural and food chemistry 51 (19): 5768–5773. doi:10.1021/jf0341767. ISSN 0021-8561. PMID 12952431.
- ^ Helmut Fiege, Heinz-Werner Voges, Toshikazu Hamamoto, Sumio Umemura, Tadao Iwata, Hisaya Miki, Yasuhiro Fujita, Hans-Josef Buysch, Dorothea Garbe, Wilfried Paulus "Phenol Derivatives" Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. doi:10.1002/14356007.a19_313
- ^ R. N. Mirrington and G. I. Feutrill (1988), "Orcinol Monomethyl Ether", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6p0859; Coll. Vol. 6: 859
- ^ C. F. H. Allen and J. W. Gates, Jr. (1955), "o-Eugenol", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv30418; Coll. Vol. 3: 418
- ^ Esposito, Lawrence J.; K. Formanek, G. Kientz, F. Mauger, V. Maureaux, G. Robert, and F. Truchet (1997). "Vanillin". Kirk-Othmer Encyclopedia of Chemical Technology, 4th edition. 24. New York: John Wiley & Sons. pp. 812–825.
- ^ CRITICAL REVIEW OF THE HEALTH EFFECTS OF WOODSMOKE
- ^ Nature, Pheromones: Exploitation of gut bacteria in the locust
Categories:- Flavors
- Natural phenols
- Phenol ethers
Wikimedia Foundation. 2010.
