- Methandrostenolone
-
Methandrostenolone 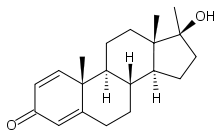
Systematic (IUPAC) name (8S,9S,10S,13S,14S,17S)-17-hydroxy-10,
13,17-trimethyl-7,8,9,11,12,14,15,16-
octahydro-6H-cyclopenta[a]phenanthren-3-oneClinical data Pregnancy cat. X(US) Legal status DEA Schedule III (US) Routes Oral Pharmacokinetic data Bioavailability Oral Metabolism Hepatic Half-life 4.5-6 hours Excretion Renal Identifiers CAS number 72-63-9 
ATC code A14AA03 PubChem CID 6300 ChemSpider 6061 
UNII COZ1R7EOCC 
ChEMBL CHEMBL1418176 
Chemical data Formula C20H28O2 Mol. mass 300.441 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Methandrostenolone (metandienone, methandienone, Averbol, Dianabol, Danabol, DBOL) is an orally-effective anabolic steroid originally developed by John Ziegler and released in the US in the early 1960s by Ciba.[1][2] It was used as an aid to muscle growth by bodybuilders until its ban by Congress under the Controlled Substances Act. However, methandrostenolone is readily available without a prescription in countries such as Mexico under the trade name Reforvit-b and is being manufactured in Asia and many East European countries, and consequently is still seen on the United States black market.
Contents
Biophysiology
Methandrostenolone doesn't react strongly with the androgen receptor but still exerts its effects through the androgen receptor in vivo.[3] These include dramatic increases in protein synthesis, glycogenolysis, and muscle strength over a short space of time. In high doses (30 mg or more per day) , side effects such as gynecomastia, high blood pressure, acne and male pattern baldness may begin to occur. The drug causes severe masculinising effects in women even at low doses. In addition, it is metabolized into methylestradiol by aromatase. This means that without the administration of aromatase inhibitors such as anastrozole or aminoglutethimide, estrogenic effects will appear over time in men. Many users will combat the estrogenic side effects with Arimidex, Nolvadex or Clomid. In addition, as with other 17α-alkylated steroids, the use of methandrostenolone over extended periods of time can result in liver damage without appropriate care.
The 17α-methylation of the steroid does allow it to pass through the liver with only a small portion of it broken down (hence causing the aforementioned damage to the liver) allowing it to be effective when taken orally. It also has the effect of decreasing the steroid's affinity for sex hormone binding globulin, a protein that de-activates steroid molecules and prevents them from further reactions with the body. As a result, methandrostenolone is significantly more active than an equivalent quantity of testosterone, resulting in rapid growth of muscle tissue. However, the concomitant elevation in estrogen levels - a result of the aromatization of methandrostenolone - results in significant water retention. This gives the appearance of great gains in mass and strength, which prove to be temporary once the steroid is discontinued and water weight drops. Because of this, it is often used by bodybuilders only at the start of a "steroid cycle", to facilitate rapid strength increases and the appearance of great size, while compounds such as testosterone or nandrolone with long acting esters build up in the body to an appreciable amount capable of supporting anabolic function on their own.
Usage
As tonic
 17α-methandrostenolone tablets confiscated by the DEA in 2008.
17α-methandrostenolone tablets confiscated by the DEA in 2008.
In the early 1960s, doctors commonly prescribed a tablet per day for women as a tonic. This use was quickly discontinued upon discovery of the heavily masculinising effects of methandrostenolone.
Bodybuilding
However, despite the lack of any known therapeutic applications, the drug remained legal until the early 1990s. The United States Congress added steroids to the Controlled Substances Act as an amendment known as the Anabolic Steroid Control Act of 1990. This act placed steroids in the same category as amphetamines as a "Schedule III" drug and possession of these drugs results in a felony. Contrary to popular belief, steroids were banned by Congress without the support of the FDA, the American Medical Association, the DEA or the National Institute on Drug Abuse.[4] Its used by bodybuilders, and methandrostenolone continues to be used illegally to this day, typically being combined (stacked) with injectable compounds, such as testosterone propionate, enanthate, cypionate as well as other injectables like trenbolone acetate.
Several successful athletes and professional bodybuilders have come forward and admitted long-term methandrostenolone use before the drug was banned, including Arnold Schwarzenegger and Sergio Oliva.[5][6] Other steroids stacked with methandrostenolone are primarily, if not always, injectable compounds such as testosterone, trenbolone and nandrolone.
Detection of use
Methandrostenolone is subject to extensive hepatic biotransformation by a variety of enzymatic pathways. The primary urinary metabolites are detectable for up to 4 days, and a recently discovered hydroxymethyl metabolite is found in urine for up to 19 days after a single 5 mg oral dose. Several of the metabolites are unique to methandrostenolone. Methods for detection in urine specimens usually involve gas chromatography-mass spectrometry.[7][8][9]
Dr. Ziegler
For a period of time Dr. Ziegler worked at the Ciba Pharmaceutical company, who supplied testosterone for experimental purposes. In the early 1950s his patients included people suffering from burns, as well as the seriously injured or handicapped. In 1954 he administered testosterone, for a period of less than 6 weeks, to several high-level competitive bodybuilders on an experimental basis, but had disappointing results. Dissatisfied and possibly overburdened with patients, he distanced himself from research into performance-enhancing drugs until May 1960, or possibly as early as 1959 (conflicting testimonials).[citation needed]
By the time of the 1960 European Championships in Milan he was understandably suspicious of the Russians - "the Russians are giving their athletes something." Therefore, he asked John Grimek to propose to his chief, Bob Hoffman that steroids be administered to members of the American Olympic team. Mr. Hoffman, however, was cautious and later remarked it was "too close to give to the men who will represent the USA". According to Grimek, "Apparently, he doesn’t think it will do that much good, and may even have detrimental effects , . . .He appears doubtful." Instead, Dianabol was given to two lower level lifters to investigate its effectiveness and safety. After that, Hoffmann retracted his decision and Dianabol was administered to certain Weightlifters on the team.[citation needed][10][11]
Footnotes
- ^ Yesalis CE, Anderson WA, Buckley WE, Wright JE (1990). "Incidence of the nonmedical use of anabolic-androgenic steroids". NIDA Res. Monogr. 102: 97–112. PMID 2079979. http://www.drugabuse.gov/pdf/monographs/102.pdf.
- ^ Fair JD (1993). "Isometrics or Steroids? Exploring New Frontiers Of Strength in the Early 1960s". Journal of Sport History 20 (1): 1–24. http://www.aafla.org/SportsLibrary/JSH/JSH1993/JSH2001/jsh2001b.pdf.
- ^ Roselli CE (May 1998). "The effect of anabolic-androgenic steroids on aromatase activity and androgen receptor binding in the rat preoptic area". Brain Res. 792 (2): 271–6. doi:10.1016/S0006-8993(98)00148-6. PMID 9593936. http://linkinghub.elsevier.com/retrieve/pii/S0006-8993(98)00148-6.
- ^ http://www.steroid.com/
- ^ Steve Theunissen: Arnold & Steroids: Truth Revealed 2002
- ^ Interview with Sergio Oliva
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 952-954.
- ^ Schänzer W, Geyer H, Fusshöller G, Halatcheva N, Kohler M, Parr MK, Guddat S, Thomas A, Thevis M. Mass spectrometric identification and characterization of a new long-term metabolite of metandienone in human urine. Rapid Commun. Mass Spectrom. 20: 2252-8, 2006.
- ^ Fragkaki AG, Angelis YS, Tsantili-Kakoulidou A, Koupparis M, Georgakopoulos C. Schemes of metabolic patterns of anabolic androgenic steroids for the estimation of metabolites of designer steroids in human urine. J. Steroid Biochem. Mol. Biol. 115: 44-61, 2009.
- ^ http://startingstrength.com/articles/ultimate_exercise_starr.pdf The Ultimate Strength Exercise 1, Bill Starr
- ^ http://startingstrength.com/articles/ultimate_exercise_2_starr.pdf The Ultimate Strength Exercise 2, Bill Starr
Other references
- Wilder EM (October 1962). "Death due to Liver Failure Following the Use of Methandrostenolone". Can Med Assoc J 87 (14): 768–9. PMC 1849648. PMID 14000685. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1849648.
- Foss GL (April 1960). "Some Experiences with a New Anabolic Steroid (Methandrostenolone)". Br Med J 1 (5182): 1300–5. doi:10.1136/bmj.1.5182.1300. PMC 1967563. PMID 13824087. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1967563.
- Zingg W (October 1965). "The Effect of Methandrostenolone on Nitrogen Excretion Following Open-Heart Surgery". Can Med Assoc J 93 (15): 816–7. PMC 1928923. PMID 5318132. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1928923.
Categories:- Anabolic steroids
Wikimedia Foundation. 2010.
