- Oxandrolone
-
Oxandrolone 
Systematic (IUPAC) name 17β-hydroxy-17α-methyl-2-oxa-5α-androstan-3-one Clinical data AHFS/Drugs.com monograph MedlinePlus a604024 Pregnancy cat. X Legal status Schedule III (US) Routes Oral Pharmacokinetic data Bioavailability 97% Metabolism Hepatic Half-life 9 hours Excretion Urinary:90%; Fecal:6% Identifiers CAS number 53-39-4 
ATC code A14AA08 PubChem CID 5878 DrugBank APRD01151 ChemSpider 5667 
UNII 7H6TM3CT4L 
KEGG D00462 
ChEBI CHEBI:7820 
ChEMBL CHEMBL1200436 
Chemical data Formula C19H30O3 Mol. mass 306.44 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Oxandrolone (Oxandrin) is a drug created by Raphael Pappo while at Searle Laboratories, now Pfizer Inc. under the trademark Anavar, and introduced into the US in 1964.
Oxandrolone is a synthetic anabolic steroid derived from dihydrotestosterone by substituting 2nd carbon atom for oxygen (O). It is widely known for its exceptionally small level of androgenicity accompanied by moderate anabolic effect. Although oxandrolone is a 17-alpha alkylated steroid, its liver toxicity is very small as well. Studies have showed that a daily dose of 20 mg oxandrolone used in the course of 12 weeks had only a negligible impact on the increase of liver enzymes[1][2]. As a DHT derivative, oxandrolone does not aromatize (convert to estrogen, which causes gynecomastia or male breast tissue). It also does not significantly influence the body's normal testosterone production (HPTA axis) at low dosages (10 mg). When dosages are high, the human body reacts by reducing the production of LH (luteinizing hormone), thinking endogenous testosterone production is too high; this in turn eliminates further stimulation of Leydig cells in the testicles, causing testicular atrophy (shrinking). Oxandrolone used in a dose of 80 mg/day suppressed endogenous testosterone by 67% after 12 weeks of therapy[1].
The drug was prescribed to promote muscle regrowth in disorders which cause involuntary weight loss. It had also been shown to be partially successful in treating cases of osteoporosis. However, in part due to bad publicity from its abuses by bodybuilders, production of Anavar was discontinued by Searle Laboratories in 1989. It was picked up by Bio-Technology General Corporation, now Savient Pharmaceuticals who, following successful clinical trials in 1995, released it under the tradename Oxandrin. As of 2009, it is the only drug marketed by the company.
It was subsequently approved for orphan drug status by the Food and Drug Administration (FDA) for treating alcoholic hepatitis, Turner syndrome, and HIV-induced weight loss. It is also indicated as an offset to protein catabolism caused by long-term administration of corticosteroids. In addition, the drug has shown positive results in treating anemia and hereditary angioedema. Because of its potential for abuse, it is categorized as a Schedule III controlled substance in the US.
In a randomized, double-blind study, patients with 40% total body surface area burns were selected to receive standard burn care plus oxandrolone, or without oxandrolone. Those treated with oxandrolone showed improved body composition, preserved muscle mass and reduced hospital stay time.[3]
Contents
Further reading
- Earthman CP, Reid PM, Harper IT, Ravussin E, Howell WH (2002). "Body cell mass repletion and improved quality of life in HIV-infected individuals receiving oxandrolone". JPEN J Parenter Enteral Nutr 26 (6): 357–65. doi:10.1177/0148607102026006357. PMID 12405647. http://pen.sagepub.com/cgi/pmidlookup?view=long&pmid=12405647.
- Hart DW, Wolf SE, Ramzy PI, et al. (April 2001). "Anabolic effects of oxandrolone after severe burn". Ann. Surg. 233 (4): 556–64. doi:10.1097/00000658-200104000-00012. PMC 1421286. PMID 11303139. http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0003-4932&volume=233&issue=4&spage=556.
- Przkora R, Herndon DN, Suman OE (January 2007). "The effects of oxandrolone and exercise on muscle mass and function in children with severe burns". Pediatrics 119 (1): e109–16. doi:10.1542/peds.2006-1548. PMC 2367234. PMID 17130281. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2367234.
- Demling RH, DeSanti L (December 2003). "Oxandrolone induced lean mass gain during recovery from severe burns is maintained after discontinuation of the anabolic steroid". Burns 29 (8): 793–7. doi:10.1016/j.burns.2003.08.003. PMID 14636753. http://linkinghub.elsevier.com/retrieve/pii/S0305417903002316.
Chemistry
http://dx.doi.org/10.1016/S0040-4039(00)70883-5
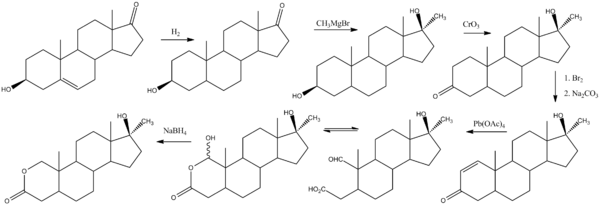
References
- ^ a b http://journals.lww.com/jaids/Fulltext/2006/03000/Oxandrolone_in_the_Treatment_of_HIV_Associated.6.aspx
- ^ http://jap.physiology.org/cgi/content/abstract/96/3/1055
- ^ Jeschke MG, Finnerty CC, Suman OE, Kulp G, Mlcak RP, Herndon DN (September 2007). "The effect of oxandrolone on the endocrinologic, inflammatory, and hypermetabolic responses during the acute phase postburn". Ann. Surg. 246 (3): 351–60; discussion 360–2. doi:10.1097/SLA.0b013e318146980e. PMC 1959346. PMID 17717439. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1959346.
External links
- Oxandrin Homepage, savientpharma.com (retrieved 23 October 2009)* Oxandrin Label, fda.gov (retrieved 23 October 2009)
- "Oxandrolone Side Effects, Interactions and Information". drugs.com. http://www.drugs.com/MTM/oxandrolone.html.
Categories:- Anabolic steroids
- Lactones
Wikimedia Foundation. 2010.
