- Trimethylaluminium
-
Trimethylaluminium 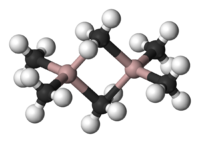 trimethylalumaneOther namesTrimethylaluminium; aluminium trimethyl
trimethylalumaneOther namesTrimethylaluminium; aluminium trimethylIdentifiers CAS number 75-24-1 
PubChem 16682925 ChemSpider 10606585 
Jmol-3D images Image 1 - C[Al](C)C
Properties Molecular formula C6H18Al2 Molar mass 144.18 g/mol Appearance Colorless liquid Density 0.752 g/mL Melting point 15 °C
Boiling point 125 °C
Hazards Main hazards Pyrophoric NFPA 704  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Trimethylaluminium is the chemical compound with the formula Al2(CH3)6, abbreviated as Al2Me6, (AlMe3)2 or the abbreviation TMA. This pyrophoric, colorless liquid is an industrially important organoaluminium compound. It evolves white smoke (aluminium oxides) when the vapor is released into the air.
Contents
Structure and bonding
Al2Me6 exists as a dimer, analogous in structure and bonding to diborane. As with diborane, the metalloids are connected by a 3-center-2-electron bond: the shared methyl groups bridge between the two aluminium atoms. The Al-C(terminal) and Al-C(bridging) distances are 1.97 and 2.14 Å, respectively.[1] The carbon atoms of the bridging methyl groups are each surrounded by five neighbors: three hydrogen atoms and two aluminium atoms. The methyl groups interchange readily intramolecularly and intermolecularly.
3-Centered-2-electron bonds are an utterance of "electron-deficient" molecules and tend to reactions with Lewis bases that would give products consisting of 2-centered-2-electron bonds. For example upon treatment with amines gives adducts R3N-AlMe3. Another reaction that gives products that follow the octet rule is the reaction of Al2Me6 with aluminium trichloride to give (AlMe2Cl)2.
The species AlMe3, which would feature an aluminium atom bonded to three methyl groups is unknown. VSEPR Theory predicts that such a molecule would have idealized threefold symmetry, as observed in BMe3.
Synthesis and applications
TMA is prepared via a two-step process that can be summarized as follows:
- 2 Al + 6 CH3Cl + 6 Na → Al2(CH3)6 + 6 NaCl
TMA is mainly used for the production of methylaluminoxane, an activator for Ziegler-Natta catalysts for olefin polymerisation. TMA is also employed as a methylation agent. Tebbe's reagent, which is used for the methylenation of esters and ketones, is prepared from TMA. TMA is often released from sounding rockets as a tracer in studies of upper atmospheric wind patterns.
TMA is also used in semiconductor fabrication to grow thin film, high-k dielectrics such as Al2O3 via the processes of Chemical Vapor Deposition or Atomic Layer Deposition.
TMA forms a complex with the tertiary amine DABCO, which is safer to handle than TMA itself.[2]
In combination with Cp2ZrCl2 (zirconocene dichloride), the (CH3)2Al-CH3 adds "across" alkynes to give vinyl aluminium species that are useful in organic synthesis in a reaction known as carboalumination.[3]
Semiconductor grade TMA
TMA is the preferred metalorganic source for metalorganic vapour phase epitaxy (MOVPE) of aluminium-containing compound semiconductors, such as AlAs, AlN, AlP, AlSb, AlGaAs, AlInGaAs, AlInGaP, AlGaN, AlInGaN, AlInGaNP etc. Criteria for TMA quality focus on (a) elemental impurites, (b) oxygenated and organic impurities.
References
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Biswas, K.; Prieto, O.; Goldsmith, P. J.Woodward, S. (2005). "Remarkably Stable (Me3Al)2DABCO and Stereoselective Nickel-Catalyzed AlR3 (R = Me, Et) Additions to Aldehydes". Angewandte Chemie International Edition 44 (15): 2232–2234. doi:10.1002/anie.200462569. PMID 15768433.
- ^ Negishi, E.; Matsushita, H., "Palladium-Catalyzed Synthesis of 1,4-Dienes by Allylation of Alkenyalane: α-Farnesene [1,3,6,10-Dodecatetraene, 3,7,11-trimethyl-]", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv7p0245; Coll. Vol. 7: 245
External links
Categories:- Organoaluminium compounds
- Coordination compounds
Wikimedia Foundation. 2010.

