- Trimethylborane
-
Trimethylborane 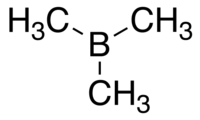 TrimethylboraneOther namestrimethylborine, trimethylboron
TrimethylboraneOther namestrimethylborine, trimethylboronIdentifiers CAS number 593-90-8 
ChemSpider 62201 
EC number 209-816-3 Jmol-3D images Image 1 - CB(C)C
Properties Molecular formula C3H9B Molar mass 55.92 g/mol Appearance Colorless gas or liquid Density 0.625 g/cm3 at -100 °C[1] Melting point -161.5 °C, 112 K, -259 °F
Boiling point -20.2 °C, 253 K, -4 °F
Solubility in water slight, Highly reactive Structure Molecular shape Δ Hazards R-phrases R17 R34[2] S-phrases S7 S23 S26 S36/37/39 S43 S45[2] Main hazards Spontaneously flammable in air; causes burns Autoignition
temperature-40 °C[2] Related compounds Related compounds triethylborane
Diborane
methylborane (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Trimethylborane is a toxic compound normally occurring as a gas that spontaneously catches fire in air. The formula is B(CH3)3, which can also be expressed as Me3B, with Me representing methyl. Its melting point is -161.5 °C and boiling point is -20.2 °C. Vapour pressure is given by log P = 6.1385+1.75 log T - 1393.3/T - 0.007735 T. T is temperature in Kelvin.[3] CAS number is 593-90-8.[4] Molecular weight is 55.914. The heat of vapourisation is 25.6 kJ/mol.[2]
Contents
Properties
As a liquid it is colourless. The strongest line in the infrared spectrum is at 1330 cm-1 followed by lines at 3010 1185 cm-1.
Preparation
Trimethylborane was first made by Stock and Zeidler. Their method of preparation combined boron trichloride gas with dimethylzinc.[3] Although the substance can be prepared using Grignard reagents the output is contaminated by unwanted products from the solvent. Trimethylborane can be made on a small scale with a 98% yield by reacting trimethylaluminium in hexane with boron tribromide in dibutyl ether as a solvent.[3] Yet other methods are reacting tributyl borate with trimethylaluminium chloride, or potassium tetrafluoroborate with trimethylaluminium.[5] Yet another method is to add boron trifluoride in ether to methyl magnesium iodide.[6]
Reactions
Trimethylborane spontaneously ignites in air if the concentration is high enough. It burns with a green flame producing soot.[7]
Trimethylborane is a strong Lewis acid. It reacts with water and chlorine at room temperature. It also reacts with grease but not with Teflon or glass.[3] Trimethylborane can form an adduct with ammonia: (NH3):B(CH3)3.[8]
Trimethylborane reacts with diborane to disproportionate to form monomethyldiborane and dimethyldiborane: (CH3)BH2.BH3 and (CH3)2BH.BH3.
It reacts as a gas with trimethylphosphine to form a solid Lewis salt with a heat of formation of -41 kcal per mol. This adduct has a heat of sublimation of -24.6 kcal/mol. No reaction occurs with trimethylarsine or trimethylstibine.[6]
Methyl lithium reacting with the Trimethylborane produces a tetramethylborate salt: LiB(CH3)4.[9] The tetramethylborate ion has a negative charge and is isoelectronic with neopentane.
Use
Trimethylborane has been used as a neutron counter. For this use it has to be very pure.[8] It is also used in chemical vapour deposition where boron and carbon need to be deposited together.
References
- ^ http://www.voltaix.com/images/doc/Msb000_TMB.pdf MSDS from Voltaix
- ^ a b c d Trimethylborane
- ^ a b c d William S. Rees, Jr. and al (1990). Alvin P. Ginsberg. ed. Trimethylborane. 27. p. 339.
- ^ Graner, G., Hirota, E., Iijima, T., Kuchitsu, K., Ramsay, D. A., Vogt, J., Vogt, N.. "C3H9B Trimethylborane". SpringerMaterials 25C. doi:10.1007/10688787_381.
- ^ Roland Koumlstera, Paul Bingera Wilhelm, V. Dahlhof (1973). "A Convenient Preparation of Trimethylborane and Triethylborane". Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry 3 (4): 359–367. doi:10.1080/00945717308057281. http://www.informaworld.com/smpp/content~db=all~content=a762676557.
- ^ a b Donald Charles Mente (May 1975). "The Reactions of Trimethyl group Va Lewis Bases with simple Boron Lewis Acids". http://etd.lib.ttu.edu/theses/available/etd-04102009-31295015069817/unrestricted/31295015069817.pdf.
- ^ Herbert Ellern (1968). "Military and Civilian Pyrotechnics". Chemical Publishing Company. p. 24. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.137.1104&rep=rep1&type=pdf.
- ^ a b Gaylon S. Ross et al (2 October 1961). "Preparation of High Purity Trimethylborane". Journal of Research of the National Bureau of Standards Physics and Chemistry 66 (1). http://nvl.nist.gov/pub/nistpubs/jres/066/1/V66.N01.A06.pdf.
- ^ Georg Wittig in 1958
Categories:- Organoboranes
- Gases
Wikimedia Foundation. 2010.
