- Samarium(III) chloride
-
Samarium(III) chloride 
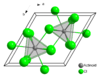
 Samarium(III) chloride
Samarium(III) chlorideIdentifiers CAS number 10361-82-7 (anhydrous)  , 13465-55-9 (hexahydrate)
, 13465-55-9 (hexahydrate)ChemSpider 55428 
Jmol-3D images Image 1 - Cl[Sm](Cl)Cl
Properties Molecular formula SmCl3 Molar mass 256.76 g/mol (anhydrous)
364.80 g/mol (hexahydrate)Appearance pale yellow solid (anhydrous) cream-coloured solid (hexahydrate)
Density 4.46 g/cm3 (anhydrous) 2.383 g/cm3 (hexahydrate)
Melting point 682 °C
Boiling point decomposes
Solubility in water 92.4 g/100 mL (10 °C) Structure Crystal structure hexagonal, hP8 Space group P63/m, No. 176 Coordination
geometryTricapped trigonal prismatic
(nine-coordinate)Hazards Main hazards Irritant  chloride (verify) (what is:
chloride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Samarium(III) chloride (SmCl3), also known as samarium trichloride, is a compound of samarium and chlorine. It is a pale yellow solid which rapidly absorbs water on exposure to moist air to form a hexahydrate, SmCl3.6H2O. Simple rapid heating of the hydrate alone may cause small amounts of hydrolysis.[1] The first five moles of water are lost at 110 °C.[2]
Contents
Chemical properties
Samarium(III) chloride is a moderately strong Lewis acid, which ranks as "hard" according to the HSAB concept. Aqueous solutions of samarium chloride can be used to prepare insoluble samarium(III) compounds, for example samarium(III) hydroxide or samarium(III) fluoride:
- SmCl3(aq) + 3 NaOH(aq) → Sm(OH)3(s) + 3 NaCl(aq)
- SmCl3(aq) + 3 KF(aq) → SmF3(s) + 3 KCl(aq)
Preparation
Samarium(III) chloride can be prepared as a yellow aqueous solution by reaction of either samarium metal or samarium(III) carbonate and hydrochloric acid. The anhydrous halide may alternatively be prepared from samarium metal and hydrogen chloride.[3][4]
- 2 Sm(s) + 6 HCl(aq) → 2 SmCl3(aq) + 3 H2(g)
Anhydrous SmCl3 can be made by dehydration of the hydrate either by slowly heating to 400 °C with 4-6 equivalents of ammonium chloride under high vacuum,[1][5][6] or by heating with an excess of thionyl chloride for five hours.[1][7] The anhydrous halide may alternatively be prepared from samarium metal and hydrogen chloride.[3][4] It is usually purified by high temperature sublimation under high vacuum.[1]
Uses
Samarium(III) chloride is used for the preparation of samarium metal (which has a variety of uses, notably in magnets). Anhydrous SmCl3 is mixed with sodium chloride or calcium chloride to lower the melting point, then it is melted and electrolysed to give the free metal.[8] The anhydrous chloride may also be used to prepare organometallic compounds of samarium, such as bis(pentamethylcyclopentadienyl)alkylsamarium(III) complexes used as catalysts for hydrogenation and hydrosilylation of alkenes.[9] Samarium(III) chloride can also be used as a starting point for the preparation of other samarium salts.
References
- ^ a b c d F. T. Edelmann, P. Poremba (1997). W. A. Herrmann. ed. Synthetic Methods of Organometallic and Inorganic Chemistry. 6. Stuttgart: Georg Thieme Verlag.
- ^ CRC Handbook of Chemistry and Physics (58th edition), CRC Press, West Palm Beach, Florida, 1977.
- ^ a b L. F. Druding, J. D. Corbett (1961). J. Am. Chem. Soc. 83 (11): 2462. doi:10.1021/ja01472a010.
- ^ a b J. D. Corbett (1973). Rev. Chim. Minerale 10: 239.
- ^ M. D. Taylor, P. C. Carter (1962). "Preparation of anhydrous lanthanide halides, especially iodides". J. Inorg. Nucl. Chem. 24 (4): 387. doi:10.1016/0022-1902(62)80034-7.
- ^ J. Kutscher, A. Schneider, (1971). "Notiz zur Präparation von wasserfreien Lanthaniden-Haloge-niden, Insbesondere von Jodiden". Inorg. Nucl. Chem. Lett. 7 (9): 815. doi:10.1016/0020-1650(71)80253-2.
- ^ J. H. Freeman, M. L. Smith (1958). "The preparation of anhydrous inorganic chlorides by dehydration with thionyl chloride". J. Inorg. Nucl. Chem. 7 (3): 224. doi:10.1016/0022-1902(58)80073-1.
- ^ Greenwood, Norman N.; Earnshaw, A. (1984). Chemistry of the Elements. Oxford: Pergamon. ISBN 0-08-022057-6.
- ^ G. A. Molander, E. D. Dowdy (1999). Shu Kobayashi. ed. Lanthanides: Chemistry and Use in Organic Synthesis. Berlin: Springer-Verlag. pp. 119–154. ISBN 3540645268.
Samarium compounds SmCl2 · SmCl3 · Categories:
- Chlorides
- Samarium compounds
- Metal halides
Wikimedia Foundation. 2010.
Look at other dictionaries:
Americium(III) chloride — chembox new ImageFile = ImageSize = IUPACName = Americium(III) chloride OtherNames = Americium chloride, americium trichloride Section1 = Chembox Identifiers Abbreviations = CASNo = 13464 46 5 EINECS = PubChem = SMILES = InChI = RTECS = MeSHName … Wikipedia
Europium(III) chloride — Europium(III) chloride … Wikipedia
Samarium — promethium ← samarium → europium ↑ Sm ↓ … Wikipedia
Samarium(II) iodide — Chembox new Name = Samarium(II) iodide ImageFile = Diiodopenta(THF)samarium(II) 3D balls.png ImageName = Ball and stick model of a samarium(II) iodide THF complex OtherNames = Samarium diiodide Name = Safety data Section1 = Chembox Identifiers… … Wikipedia
Plutonium(III) fluoride — chembox new ImageFile = ImageSize = IUPACName = Plutonium(III) fluoride OtherNames = Plutonium fluoride, plutonium trifluoride Section1 = Chembox Identifiers Abbreviations = CASNo = 13842 83 6 EINECS = PubChem = SMILES = InChI = RTECS = MeSHName … Wikipedia
List of inorganic compounds — Tentative listing related to this page, inorganic compounds by element (presently under construction), as well as . This list is not necessarily complete or up to date ndash; if you see an article that should be here but isn t (or one that… … Wikipedia
Dictionary of chemical formulas — This is a list of chemical compounds with chemical formulas and CAS numbers, indexed by formula. This complements alternative listings to be found at list of inorganic compounds, list of organic compounds and inorganic compounds by element. Table … Wikipedia
Cobalt — This article is about the metal. For other uses, see Cobalt (disambiguation). iron ← cobalt → nickel ↑ Co ↓ Rh … Wikipedia
Reduction of nitro compounds — The chemical reactions described as reduction of nitro compounds can be facilitated by many different reagents and reaction conditions. Historically, the nitro group was one of the first functional groups to be reduced, due to the ease of nitro… … Wikipedia
Friedel–Crafts reaction — The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877.[1] There are two main types of Friedel–Crafts reactions: alkylation reactions and acylation reactions. This reaction type is a form of… … Wikipedia
