- Europium(III) chloride
-
Europium(III) chloride 
 Europium(III) chloride
Europium(III) chloride
Europium trichlorideIdentifiers CAS number 10025-76-0  ,
,
13759-92-7 (hexahydrate)ChemSpider 23194 
EC number 233-040-4 RTECS number LE7525000 Jmol-3D images Image 1 - Cl[Eu](Cl)Cl
Properties Molecular formula EuCl3 Molar mass 258.32 g/mol
366.41 g/mol (hexahydrate)Melting point 632 °C decomp.
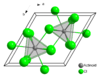
Solubility in other solvents Soluble Related compounds Related compounds Europium dichloride Structure Crystal structure hexagonal (UCl3 type), hP8 Space group P63/m, No. 176 Coordination
geometryTricapped trigonal prismatic
(nine-coordinate) chloride (verify) (what is:
chloride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Europium(III) chloride is a compound of europium and chlorine with the formula EuCl3.
Contents
Properties
Europium trichloride is a yellow solid which begins to decompose at or below its melting point to give at least some hygroscopic it rapidly absorbs water on exposure to moist air to form a white crystalline hexahydrate, EuCl3·6H2O. Simple rapid heating of the hydrate alone may cause small amounts of hydrolysis. Anhydrous EuCl3 can be made from the hydrate by heating with an excess of thionyl chloride for around 15 hours.[1]
Europium(III) chloride is soluble in water. When anhydrous, it is expected to be also highly soluble in ethanol (by analogy with SmCl3). It is nine-coordinate (trigonal prismatic), and it crystallises with the UCl3 structure.[2]
Uses
Europium(III) chloride can be used for the preparation of europium(II) chloride by reduction in a gold boat using hydrogen gas while heating slowly to 700 °C. The anhydrous chloride may also be used to prepare organometallic compounds of europium, such as bis(pentamethylcyclopentadienyl)europium(II) complexes.[3][4] Europium(III) chloride can be used as a starting point for the preparation of other europium salts.
Bibliography
- Edelmann, F. T.; & Poremba, P. (1997). in: Synthetic Methods of Organometallic and Inorganic Chemistry (Herrmann, E. A., Ed.) Vol. 6. Stuttgart:Georg Thieme.
- Weast, R. C., ed (1972). Handbook of Chemistry and Physics (53rd ed.). Cleveland, OH: Chemical Rubber Co..
References
- ^ Freeman, J.H.; Smith, M.L. (1958). "Preparation of Anhydrous Inorganic Chlorides by Dehydration with Thionyl Chloride". J. Inorg. Nucl. Chem. 7 (3): 224–227. doi:10.1016/0022-1902(58)80073-1.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 0080379419.
- ^ Tilley, T. Don; Andersen, Richard A.; Spencer, Brock; Ruben, Helena; Zalkin, Allan; Templeton, David H. (1980). "Divalent lanthanide chemistry. Bis (pentamethylcyclopentadienyl) europium(II) and -ytterbium(II) derivatives: crystal structure of bis (pentamethylcyclopentadienyl) (tetrahydrofuran ytterbium(II) -hemitoluene at 176 K". Inorganic Chemistry 19 (10): 2999. doi:10.1021/ic50212a031.
- ^ Evans, William J.; Hughes, Laura A.; Hanusa, Timothy P. (1986). "Synthesis and x-ray crystal structure of bis(pentamethylcyclopentadienyl) complexes of samarium and europium: (C5Me5)2Sm and (C5Me5)2Eu". Organometallics 5 (7): 1285. doi:10.1021/om00138a001.
Europium compounds Categories:- Chlorides
- Europium compounds
- Metal halides
Wikimedia Foundation. 2010.
