- Chitosan
-
Chitosan 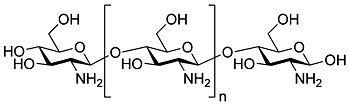 Other namesPoliglusam; Deacetylchitin; Poly-(D)glucosamine
Other namesPoliglusam; Deacetylchitin; Poly-(D)glucosamineIdentifiers CAS number 9012-76-4 
ChemSpider 64870 
Jmol-3D images Image 1 - COC(=O)N[C@@H]1[C@H]([C@@H]([C@H](O[C@H]1O[C@@H]2[C@H](O[C@H]([C@@H]([C@H]2O)N)O[C@@H]3[C@H](O[C@H]([C@@H]([C@H]3O)N)O)CO)CO)CO)O[C@H]4[C@@H]([C@H]([C@@H]([C@H](O4)CO)O[C@H]5[C@@H]([C@H]([C@@H]([C@H](O5)CO)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O[C@H]7[C@@H]([C@H]([C@@H]([C@H](O7)CO)O[C@H]8[C@@H]([C@H]([C@@H]([C@H](O8)CO)O[C@H]9[C@@H]([C@H]([C@@H]([C@H](O9)CO)O)O)N)O)N)O)N)O)N)O)N)O)N)O
- InChI=1S/C56H103N9O39/c1-87-56(86)65-28-38(84)46(19(10-74)96-55(28)104-45-18(9-73)95-49(27(64)37(45)83)97-39-12(3-67)88-47(85)20(57)31(39)77)103-54-26(63)36(82)44(17(8-72)94-54)102-53-25(62)35(81)43(16(7-71)93-53)101-52-24(61)34(80)42(15(6-70)92-52)100-51-23(60)33(79)41(14(5-69)91-51)99-50-22(59)32(78)40(13(4-68)90-50)98-48-21(58)30(76)29(75)11(2-66)89-48/h11-55,66-85H,2-10,57-64H2,1H3,(H,65,86)/t11-,12-,13-,14-,15-,16-,17-,18-,19-,20-,21-,22-,23-,24-,25-,26-,27-,28-,29-,30-,31-,32-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48+,49+,50+,51+,52+,53+,54+,55+/m1/s1

Key: FLASNYPZGWUPSU-SICDJOISSA-N InChI=1/C56H103N9O39/c1-87-56(86)65-28-38(84)46(19(10-74)96-55(28)104-45-18(9-73)95-49(27(64)37(45)83)97-39-12(3-67)88-47(85)20(57)31(39)77)103-54-26(63)36(82)44(17(8-72)94-54)102-53-25(62)35(81)43(16(7-71)93-53)101-52-24(61)34(80)42(15(6-70)92-52)100-51-23(60)33(79)41(14(5-69)91-51)99-50-22(59)32(78)40(13(4-68)90-50)98-48-21(58)30(76)29(75)11(2-66)89-48/h11-55,66-85H,2-10,57-64H2,1H3,(H,65,86)/t11-,12-,13-,14-,15-,16-,17-,18-,19-,20-,21-,22-,23-,24-,25-,26-,27-,28-,29-,30-,31-,32-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48+,49+,50+,51+,52+,53+,54+,55+/m1/s1
InChI=1/C56H103N9O39/c1-87-56(86)65-28-38(84)46(19(10-74)96-55(28)104-45-18(9-73)95-49(27(64)37(45)83)97-39-12(3-67)88-47(85)20(57)31(39)77)103-54-26(63)36(82)44(17(8-72)94-54)102-53-25(62)35(81)43(16(7-71)93-53)101-52-24(61)34(80)42(15(6-70)92-52)100-51-23(60)33(79)41(14(5-69)91-51)99-50-22(59)32(78)40(13(4-68)90-50)98-48-21(58)30(76)29(75)11(2-66)89-48/h11-55,66-85H,2-10,57-64H2,1H3,(H,65,86)/t11-,12-,13-,14-,15-,16-,17-,18-,19-,20-,21-,22-,23-,24-,25-,26-,27-,28-,29-,30-,31-,32-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48+,49+,50+,51+,52+,53+,54+,55+/m1/s1
Key: FLASNYPZGWUPSU-SICDJOISBY
Related compounds Related compounds D-glucosamine and N-Acetylglucosamine (monomers)  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Chitosan (
 /ˈkaɪtɵsæn/) is a linear polysaccharide composed of randomly distributed β-(1-4)-linked D-glucosamine (deacetylated unit) and N-acetyl-D-glucosamine (acetylated unit). It has a number of commercial and possible biomedical uses.
/ˈkaɪtɵsæn/) is a linear polysaccharide composed of randomly distributed β-(1-4)-linked D-glucosamine (deacetylated unit) and N-acetyl-D-glucosamine (acetylated unit). It has a number of commercial and possible biomedical uses.Contents
Manufacture and properties
 Commercial chitosan is derived from the shells of shrimp and other sea crustaceans, including Pandalus borealis, pictured here.[1]
Commercial chitosan is derived from the shells of shrimp and other sea crustaceans, including Pandalus borealis, pictured here.[1]
Chitosan is produced commercially by deacetylation of chitin, which is the structural element in the exoskeleton of crustaceans (such as crabs and shrimp) and cell walls of fungi. The degree of deacetylation (%DD) can be determined by NMR spectroscopy, and the %DD in commercial chitosans ranges from 60 to 100%. On average, the molecular weight of commercially produced chitosan is between 3800 and 20,000 Daltons. A common method for the synthesis of chitosan is the deacetylation of chitin using sodium hydroxide in excess as a reagent and water as a solvent. This reaction pathway, when allowed to go to completion (complete deacetylation) yields up to 98% product.[2]
The amino group in chitosan has a pKa value of ~6.5, which leads to a protonation in acidic to neutral solution with a charge density dependent on pH and the %DA-value. This makes chitosan water soluble and a bioadhesive which readily binds to negatively charged surfaces such as mucosal membranes. Chitosan enhances the transport of polar drugs across epithelial surfaces, and is biocompatible and biodegradable. Purified quantities of chitosans are available for biomedical applications.
Chitosan and its derivatives, such as trimethylchitosan (where the amino group has been trimethylated), have been used in nonviral gene delivery. Trimethylchitosan, or quaternised chitosan, has been shown to transfect breast cancer cells, with increased degree of trimethylation increasing the cytotoxicity; at approximately 50% trimethylation, the derivative is the most efficient at gene delivery. Oligomeric derivatives (3-6 kDa) are relatively nontoxic and have good gene delivery properties.[3]
Usage
Agricultural and horticultural use
Natural biocontrol and elicitor
In agriculture, chitosan is used primarily as a natural seed treatment and plant growth enhancer, and as an ecologically friendly biopesticide substance that boosts the innate ability of plants to defend themselves against fungal infections.[4] The natural biocontrol active ingredients, chitin/chitosan, are found in the shells of crustaceans, such as lobsters, crabs, and shrimp, and many other organisms, including insects and fungi. It is one of the most abundant biodegradable materials in the world. Degraded molecules of chitin/chitosan exist in soil and water. Chitosan applications for plants and crops are regulated by the EPA, and the USDA National Organic Program regulates its use on organic certified farms and crops.[5] EPA-approved, biodegradable chitosan products are allowed for use outdoors and indoors on plants and crops grown commercially and by consumers.[6] The natural biocontrol ability of chitosan should not be confused with the effects of fertilizers or pesticides upon plants or the environment. Chitosan active biopesticides represent a new tier of cost-effective biological control of crops for agriculture and horticulture.[7] The biocontrol mode of action of chitosan elicits natural innate defense responses within plant to resist insects, pathogens, and soil-borne diseases when applied to foliage or the soil.[8] Chitosan increases photosynthesis, promotes and enhances plant growth, stimulates nutrient uptake, increases germination and sprouting, and boosts plant vigor. When used as seed treatment or seed coating on cotton, corn, seed potatoes, soybeans, sugar beets, tomatoes, wheat and many other seeds, it elicits an innate immunity response in developing roots which destroys parasitic cyst nematodes without harming beneficial nematodes and organisms.[9][10] Agricultural applications of chitosan can reduce environmental stress due to drought and soil deficiencies, strengthen seed vitality, improve stand quality, increase yields, and reduce fruit decay of vegetables, fruits and citrus crops (see photo right).[11] Horticultural applications of chitosan increases blooms and extends the life of cut flowers and Christmas trees.[12] The US Forest Service has conducted research on chitosan to control pathogens in pine trees.[13][14] and chitosan's ability to increase pine tree resin pitch outflow by 40% to resist pine beetle infestation.[15]
 NASA life support GAP technology with untreated beans (left tube) and ODC chitosan biocontrol-treated beans (right tube) returned from the Mir space station aboard the space shuttle – September 1997
NASA life support GAP technology with untreated beans (left tube) and ODC chitosan biocontrol-treated beans (right tube) returned from the Mir space station aboard the space shuttle – September 1997Chitosan has a rich history of being researched for applications in agriculture and horticulture dating back to the 1980s.[16] By 1989, Bentech Labs patented chitosan salt solutions applied to crops for improved freeze protection or to crop seed for seed priming.[17] Shortly thereafter, Bentech's chitosan salt received the first ever biopesticide label from the EPA. Numerous other chitosan patents for plants soon followed. Chitosan applications to protect plants have been used in space, as well. NASA first flew a chitosan experiment to protect adzuki beans grown aboard the space shuttle and Mir space station in 1997 (see photo left).[18] NASA results revealed chitosan induces increased growth (biomass) and pathogen resistance due to elevated levels of beta 1-3 glucanase enzymes within plant cells. NASA confirmed chitosan elicits the same effect in plants on earth.[19] Over 20 years of research and development by DuPont/ConAgra Ventures (DCV) and AgriHouse, Inc have gone into developing nontoxic, low molecular weight chitosan polymer solutions safe enough for broad-spectrum agricultural and horticultural use.[20][21] In 2008, AgriHouse, Inc, Denver (Berthoud), Colorado, was granted EPA natural broad-spectrum elicitor status for YEA! Yield Enhancing Agent, a liquid solution containing an ultralow molecular active ingredient of 0.25% chitosan.[22] YEA! is a next-generation, natural chitosan elicitor solution for agriculture and horticultural uses, and was granted an amended label for foliar and irrigation applications by the EPA in June, 2009. A milliliter of YEA! contains over 14.4 X 10¹³ bioactive, low molecular weight chitosan molecules, and it is 600 times more effective than common chitosan.[23] Given its low potential for toxicity and its abundance in the natural environment, chitosan does not harm people, pets, wildlife, or the environment when used according to label directions.[24] Agricultural chitosan facts are located on USDA and EPA web sites.[25][26]
The US Forest Service tested chitosan as an ecofriendly biopesticide, to prearm pine trees to defend themselves against mountain pine beetles.
Water filtration
Chitosan can also be used in water processing engineering as a part of a filtration process. Chitosan causes the fine sediment particles to bind together, and is subsequently removed with the sediment during sand filtration. It also removes phosphorus, heavy minerals, and oils from the water. Chitosan is an important additive in the filtration process. Sand filtration apparently can remove up to 50% of the turbidity alone, while the chitosan with sand filtration removes up to 99% turbidity.[27] Chitosan has been used to precipitate caseins from bovine milk and cheese making. [1][2]
Chitosan is also useful in other filtration situations, where one may need to remove suspended particles from a liquid. In combination with bentonite, gelatin, silica gel, isinglass, or other fining agents, it is used to clarify wine, mead, and beer. Added late in the brewing process, chitosan improves flocculation, and removes yeast cells, fruit particles, and other detritus that cause hazy wine. Chitosan combined with colloidal silica is becoming a popular fining agent for white wines, because chitosan does not require acidic tannins (found primarily in red wines) with which to flocculate.[28]
Potential industrial use
Scientists have recently developed a polyurethane coating that heals its own scratches when exposed to sunlight, offering the promise of scratch-free cars and other products. The self-healing coating uses chitosan incorporated into traditional polymer materials, such as those used in coatings on cars, to protect paint. When a scratch damages the chemical structure, the chitosan responds to ultraviolet light by forming chemical chains that begin bonding with other materials in the substance, eventually smoothing the scratch. The process can take less than an hour.[29]
Marek W. Urban, a scientist working on this project, said the polymer can only repair itself in the same spot once, and would not work after repeated scratches.[30] Whether this technology can be applied to industrial materials, however, depends on a number of factors (long-term persistence of "healability", stiffness and heat resistance of coating, knowledge of the exact mechanism of healing, etc.) not present initial studies; further investigation into these factors can potentially take decades to rectify.
Biomedical uses
Chitosan's properties allow it to rapidly clot blood, and has recently gained approval in the United States and Europe for use in bandages and other hemostatic agents. Chitosan hemostatic products have been shown in testing by the U.S. Marine Corps to quickly stop bleeding and to reduce blood loss, and result in 100% survival of otherwise lethal arterial wounds in swine.[31] Chitosan hemostatic products reduce blood loss in comparison to gauze dressings and increase patient survival.[32] Chitosan hemostatic products have been sold to the U.S. Army and are currently used by the UK military. Both the US and UK have already used the bandages on the battlefields of Iraq and Afghanistan.[33] Chitosan is hypoallergenic and has natural antibacterial properties, which further support its use in field bandages.[34]
Chitosan's properties also allow it to be used in transdermal drug delivery; it is mucoadhesive in nature, reactive (so it can be produced in many different forms), and most importantly, its positive charge under acidic conditions. This positive charge comes from protonation of its free amino groups. Lack of a positive charge means chitosan is insoluble in neutral and basic environments. However, in acidic environments, protonation of the amino groups leads to an increase in solubility. The implications of this are very important to biomedical applications. This molecule will maintain its structure in a neutral environment, but will solubilize and degrade in an acidic environment. This means chitosan can be used to transport a drug to an acidic environment, where the chitosan packaging will then degrade, releasing the drug to the desired environment. One example of this drug delivery has been the transport of insulin.[35]
Claimed health benefits
Chitosan is frequently sold as tablet form at health stores as a "fat binder": It is supposed to have the capability to interact with lipids (fat) from the digestive system and limit their absorption in the body. Therefore, chitosan can be an effective complement to help lose weight during diet period or to stabilise one's weight. In the 2007 Cochrane meta-analysis,[36] which evaluated all available clinical trials performed with chitosan on the subject of weight loss, body weight and all parameters related to cholesterol changed in favor of chitosan compared to placebo. The mean difference in body weight was −1.7 kg (range: −2.1 to −1.3) in favor of chitosan, which was statistically significant. There was no difference between chitosan and placebo concerning side effects. The various qualities (in terms of duration, sample size, doses, subject characteristics, type of diet, chitosan quality and characteristics, etc.) of the clinical trials performed to evaluate the effect of chitosan on body weight might account for some of the disparities observed in clinical trial results,[37] and the subsequent critics regarding the real efficacy of chitosan. In an experimental model of the stomach and duodenum tract, chitosan has shown to interact with oil, which inhibited duodenal absorption and enhanced lipid excretion.[38] However, the mechanism of interaction between chitosan and fat is not very well understood and has not been proved clinically yet.[39] Because of this, the FDA has issued in 2004 Warning Letters to two companies who made inappropriate claims, according to the regulator.[40] Although detractors claim using chitosan may have the deleterious effect of rendering ineffective certain minerals found in foodstuffs, several animal studies contradicted this statement by showing no or little effect. In mice, dietary ingestion of chitosan did not depress the level of iron, zinc or copper.[41] Moreover, there is no proof of any adverse events, in particular regarding nutrient absorption, in humans.
Medical research
Chitosan is currently the focus of much medical research, as it is a polyglucosamine (the second-most-common dietary fiber, after cellulose).[42] Studies have shown chitosan has the following properties:
- As a soluble dietary fiber, it increases gastrointestinal lumen viscosity and slows down the emptying of the stomach.
- It alters bile acid composition, increasing the excretion of sterols and reducing the digestibility of ileal fats.[43][44][45] It is unclear how chitosan does this, but the currently favored hypotheses involve the increase of intestinal viscosity or bile acid-binding capacity.[46]
- Chitosan is relatively insoluble in water, but can be dissolved by dilute acids, which would make it a highly-viscous dietary fiber.[46] Such fibers might inhibit the uptake of dietary lipids by increasing the thickness of the boundary layer of the intestinal lumen, which has been observed in animal experiments.[47]
- Having very few acetyl groups, chitosan contains cationic groups. This may cause chitosan to have bile acid-binding capacity, which causes mixed micelles to be entrapped or disintegrated in the duodenum and ileum.[46] This would interrupt bile acid circulation, causing reduced lipid absorption and increased sterol excretion, which has also been observed in animal experiments.[45][46][47]
See also
References
- ^ Shahidi, F. and Synowiecki, J. (1991). "Isolation and characterization of nutrients and value-added products from snow crab (Chionoecetes opilio) and shrimp (Pandalus borealis) processing discards" (PDF). Journal of Agricultural and Food Chemistry (American Chemical Society) 39 (8): 1527–1532. doi:10.1021/jf00008a032. http://pubs.acs.org/cgi-bin/abstract.cgi/jafcau/1991/39/i08/f-pdf/f_jf00008a032.pdf.
- ^ Yuan, Zhuangdong (2007). "Study on the synthesis and catalyst oxidation properties of chitosan bound nickel(II) complexes." (PDF). Journal of Agricultural and Food Chemistry (Huagong Shikan Zazhishe) 21 (5): 22–24.
- ^ Kean T, Roth S, Thanou M (2005). "Trimethylated chitosans as non-viral gene delivery vectors: cytotoxicity and transfection efficiency". J Control Release 103 (3): 643–53. doi:10.1016/j.jconrel.2005.01.001. PMID 15820411.
- ^ "Linden, J., Stoner, R., Knutson, K. Gardner-Hughes, C. "Organic Disease Control Elicitors". Agro Food Industry Hi-Te (p12-15 Oct 2000)". http://www.yeacrops.com/Crop%20Protection%20Article.pdf.
- ^ "USDA NOP and EPA Rule on Chitosan, Federal Register/Vol. 72, No. 236/Monday, December 10, 2007/Rules and Regulation". http://74.125.95.132/search?q=cache:YOkqBxU7-D0J:edocket.access.gpo.gov/2007/pdf/E7-23831.pdf+chitosan+EPA&cd=10&hl=en&ct=clnk&gl=us.
- ^ "Chitin and Chitosan Final Registration Review Decision, Document ID: EPA-HQ-OPP-2007-0566-0019, Dec 11, 2008, pp 10–15, Regulations.gov". http://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPP-2007-0566-0019.
- ^ Goosen, Mattheus F. A (1996-06-01). Goosen, M. F., 1996, Applications of Chitin and Chitosan, pp 132–139, CRC Press.. ISBN 9781566764490. http://books.google.com/?id=3YmDQyVfsDkC&dq=chitosan+seed+treatment.
- ^ "Linden, J.C. and Stoner, R.J. 2005. Proprietary Elicitor Affects Seed Germination and Delays Fruit Senescence.". Journal of Food, Agriculture & Environment. http://www.yeacrops.com/Elicitor%20-%20Ethylene%20Reduction.pdf..
- ^ "Smiley R., Cook R.J., Pauliz T., Seed Treatment for Sample Cereal Grains Oregon State University, 2002, EM 8797". http://extension.oregonstate.edu/catalog/pdf/em/em8797.pdf#search=%22YEA!%20Seed%20treatment%22.
- ^ "Stoner R., Linden J., Micronutrient elicitor for treating nematodes in field crops, 2006, Patent Pending, Pub. no.: US 2008/0072494 A1". http://www.google.com/patents?id=XMeqAAAAEBAJ&dq=micronutrients+nematode+suppression.
- ^ "Linden, J.C. and Stoner, R.J. 2007. Pre-harvest application of proprietary elicitor delays fruit senescence. A. Ramina et al. (eds.). Advances in Plant Ethylene Research: Proceedings of the 7th International Symposium on the Plant Hormone Ethylene. pp 301–302. Springer: Dordrecht, The Netherlands". http://www.springerlink.com/content/uh809638432m0u04/.
- ^ "YouTube video / Chitosan Extends the Life of Plants and Trees". http://www.yeacrops.com/yeatrees.htm.
- ^ "Mason, Mary E.; Davis, John M., Defense Response in Slash Pine: Chitosan Treatment Alters the Abundance of Specific mRNAs". http://www.treesearch.fs.fed.us/pubs/672.
- ^ "Klepzig, Kier D.; Walkinshaw, Charles H., Cellular response of loblolly pine to wound inoculation with bark beetle-associated fungi and chitosan". http://www.treesearch.fs.fed.us/pubs/5322.
- ^ O'Toole, Erin (2009-09-10). "Solution for Pine Bark Beetles May Help Front Range Trees". NPR Morning Edition - KUNC 91.5 FM Greeley, CO. http://www.publicbroadcasting.net/kunc/news.newsmain/article/1/0/1552856/Regional/Solution.for.Pine.Bark.Beetles.May.Help.Front.Range.Trees.
- ^ "Croteau, R., Gurkewitz, R., Johnson, M., and Fisk, H., Monoterpene and Diterpene Biosynthesis in Lodgepole Pine Saplings Infected with Ceratocystis clavigera or Treated with Carbohydrate Elicitors, Plant Physiology 85:1123–1128(1987)". http://www.plantphysiol.org/cgi/content/abstract/85/4/1123.
- ^ "Treatment of Plants with Chitosan Salts, 1989, Patent WO/1989/007395". http://www.wipo.int/pctdb/en/wo.jsp?wo=1989007395.
- ^ "Stoner, R., Progressive Plant Growing Has Business Blooming, Environmental and Agricultural Resources NASA Spinoff 2006, pp. 68–71". http://www.nasa.gov/vision/earth/technologies/aeroponic_plants.html..
- ^ "Linden, J., Stoner, R., YEA! Elicitor Response Comparison to Chitin / Chitosan in Mung Bean and Adzuki Bean Germination Experiments, 2008". http://www.yeacrops.com/Compare%20YEA!%20to%20Chitin-chitosan.pdf.
- ^ "BIOPOLYMERS Making Materials Nature's Way". http://www.scribd.com/doc/4101166/9313.
- ^ "SeedQuest Press Release: AgriHouse Acquires DCV Chitosan IP and Patents". http://seedquest.com/yellowpages/americas/usa/a/agrihouse/default.htm.
- ^ "Chitin/Chitosan, Farnesol/Nerolidol and Nosema locustae Final Registration Review Decision; Federal Register Notice of Availability December 24, 2008 (Volume 73, Number 248) EPA". http://www.epa.gov/EPA-PEST/2008/December/Day-24/p30496.htm.
- ^ "Linden, J.C. and Stoner, R.J. 2007. Pre-harvest application of proprietary elicitor delays fruit senescence. A. Ramina et al. (eds.). Advances in Plant Ethylene Research: Proceedings of the 7th International Symposium on the Plant Hormone Ethylene. p302. Springer: Dordrecht, The Netherlands". http://www.springerlink.com/content/uh809638432m0u04/.
- ^ "Chitosan Exemption from the Requirement of a Tolerance". http://www.epa.gov/fedrgstr/EPA-PEST/1995/April/Day-19/pr-224.html.
- ^ "Control Strategies to reduce postharvest decay of fresh fruits and vegetables". http://pubsearch.arsnet.usda.gov/search?q=chitosan&requiredfields=spsite_id%3A12750000%7Cspsite_id%3A12-50-00-00&btnG=Go%21&filter=0&as_sitesearch=ars.usda.gov&ie=&output=xml_no_dtd&client=ars_frontend&proxystylesheet=ars_frontend&lr=&oe=.
- ^ "Chitosan; Poly-D-glucosamine (128930) Fact Sheet". US Environmental Protection Agency. May 2nd 2006. http://www.epa.gov/pesticides/biopesticides/ingredients/factsheets/factsheet_128930.htm. Retrieved 2006-07-10.
- ^ Alan Woodmansey (Highway Engineer) (March 19 2002). "Chitosan Treatment of Sediment Laden Water - Washington State I-90 Issaquah Project". Federal Highway Administration. U.S. Department of Transportation. http://www.fhwa.dot.gov/engineering/geotech/policymemo/tanks.cfm. Retrieved 2006-07-10.
- ^ Rayner, Terry. "Fining and Clarifying Agents". Archived from the original on June 16, 2006. http://web.archive.org/web/20060616062535/http://makewine.com/makewine/fining.html. Retrieved 2006-07-18.
- ^ Self-Repairing Oxetane-Substituted Chitosan Polyurethane Networks
- ^ Coating makes scratches on cars disappear
- ^ Journal of Emergency Medicine: 74–81. January 2008.
- ^ Pusateri, A. E., S. J. McCarthy, K. W. Gregory, R. A. Harris, L. Cardenas, A. T. McManus & C. W. Goodwin Jr. (2003). "Effect of a chitosan-based hemostatic dressing on blood loss and survival in a model of severe venous hemorrhage and hepatic injury in swine". Journal of Trauma 4 (1): 177–182. doi:10.1097/00005373-200301000-00023. PMID 12544915. http://www.jtrauma.com/pt/re/jtrauma/abstract.00005373-200301000-00023.htm.
- ^ Karen Lurie. "War Bandages". http://www.sciencentral.com/articles/view.php3?type=article&article_id=218392341.
- ^ Kevin McCue (March 3 2003). "New Bandage Uses Biopolymer" (- Scholar search). Chemistry.org (American Chemical Society). Archived from the original on November 28, 2005. http://web.archive.org/web/20051128100345/http://www.chemistry.org/portal/a/c/s/1/feature_ent.html?id=401c0f5c4d8511d7f6e36ed9fe800100. Retrieved 2006-07-10.
- ^ Sunil A. Agnihotri, Nadagouda N. Mallikarjuna and Tejraj M. Aminabhavi (2004). "Recent advances on chitosan-based micro- and nanoparticles in drug delivery" (PDF). Journal of Controlled Release (Elsevier B.V.) 100 (1): 5–28. doi:10.1016/S1097-2765(00)00071-X. PMID 11030352.
- ^ Jull AB, Ni Mhurchu C, Bennett DA, Dunshea-Mooij CAE, Rodgers A (2008). "Chitosan for overweight or obesity". Cochrane review.
- ^ Schiller RN, Barrager E, Schauss AG, Nichols EJ. (2001). "A Randomized, Double-Blind, Placebo-Controlled Study Examining the Effects of a Rapidly Soluble Chitosan Dietary Supplement on Weight Loss and Body Composition in Overweight and Mildly Obese Individuals". Journal of the American Nutraceutical Association.
- ^ Rodríguez MS, Albertengo LE. (2005). "Interaction between chitosan and oil under stomach and duodenal digestive chemical conditions". Biosci Biotechnol Biochem.
- ^ Matthew D. Gades and Judith S. Stern (2003). "Chitosan supplementation and fecal fat excretion in men". Obesityresearch.org (Obesity Research). http://www.obesityresearch.org/cgi/content/abstract/11/5/683?etoc. Retrieved 2008-02-18.
- ^ "Warning Letter for Weight Loss Products". http://google2.fda.gov/search?q=warning+letter+chitosan&client=FDAgov&site=FDAgov&lr=&proxystylesheet=FDAgov&output=xml_no_dtd&getfields=*&ie=UTF-8&ip=194.78.203.60&access=p&sort=date%3AD%3AL%3Ad1&entqr=3&oe=UTF-8&ud=1&start=0.
- ^ Zeng L, Qin C, He G, Wang W, Li W, Xu D. (2008). "Effect of dietary chitosans on trace iron, copper and zinc in mice". Carbohydrate Polymers 74.
- ^ Knorr, D. (January 1991). "Recovery and utilization of chitin and chitosan in food processing waste management". Food Technology 45 (1): 114–122.
- ^ Fukada, Y.; Kimura, K.; Ayaki, Y. (1991). "Effect of chitosan feeding on intestinal bile acid metabolism in rats". Lipids (Springer Berlin / Heidelberg) 26 (5): 395–399. doi:10.1007/BF02537206. ISSN (Print) 0024-4201 (Print). PMID 1895888. http://www.springerlink.com/content/p08107u75814t787/.
- ^ Maezaki, Y.; Tsuji, K.; Nakagawa, Y.; Kawai, Y.; Akimoto, M., et al (1993). "Hypocholesterolemic effect of chitosan in adult males". Bioscience, Biotechnology and Biochemistry 57 (9): 1439–1444. doi:10.1271/bbb.57.1439. ISSN 09168451.
- ^ a b Razdan A., Pettersson D. (1994). "Effect of chitin and chitosan on nutrient digestibility and plasma lipid concentrations in broiler chickens". British Journal of Nutrition 74 (2): 277–288. doi:10.1079/BJN19940029. PMID 7947645.
- ^ a b c d I. Furda (1990). "Interaction of dietary fiber with lipids—mechanistic theories and their limitations". Advances in Experimental Medicine and Biology (Plenum Press) 270: 67–82. ISSN (Print) 0065-2598 (Print). PMID 1964019.
- ^ a b Ikeda, I.; Sugano, M.; Yoshida, K.; Sasaki, E.; Iwamoto, Y.; Hatano, K. (March 1993). "Effects of chitosan hydrolysates on lipid absorption and on serum and liver lipid concentration in rats". Agriculture and Food Chemistry 41 (3): 431–435. doi:10.1021/jf00027a016. ISSN 0021-8561.
External links
- Crab chemical could give cars a self-healing "shell" — Video of self-healing action
- Information about chitosan properties and research and development projects (Heppe Medical Chitosan)
- Chitosan: Revolutionizing Weight Loss? — Jamie Fritch's opinion of chitosan
- The Chitosanase Web Page — dedicated to the enzymatic hydrolysis of chitosan.
- Battlefield Band-Aids — "But now, scientists have created a bandage that is actually able to clot a bullet wound in less than a minute. The bandages are laced with a mixture of ground shrimp shells and vinegar, a concoction that has been found to clot blood instantly. The key ingredient in the shrimp shells is called chitosan."
- Is Chitosan a "Fat Magnet"? — A critical look on the claims how chitosan can be used for weight management
- Crab Glue (08/05/2008) — Catalyst (an ABC-TV Program) looked at chitosan's potential as a surgical adhesive in theatre.
- Chitosan for emergency bleeding — more information on emergency bleeding products made with Chitosan.
- Absorbitol - an alternative name for Chitosan — Research led information on Absorbitol and its uses as a nutritional supplement
Categories:- Polysaccharides
- Antihemorrhagics
Wikimedia Foundation. 2010.
