- Chain growth polymerisation
-
Chain growth polymerization is a polymerization technique where unsaturated monomer molecules add on to a growing polymer chain one at a time [1]. It can be represented with the chemical equation:
where n is the degree of polymerization.
"Chain growth polymerization" and addition polymerization (also called polyaddition) are two different concepts. In fact polyurethane polymerizes with addition polymerization (because its polymerization does not produce any small molecules, called "condensate"), but its reaction mechanism is a step-growth polymerization.
The distinction between "addition polymerization" and "condensation polymerization" was introduced by Wallace Hume Carothers in 1929, and are referred to the type of products, respectively:[2][3]
- a polymer only (addition)
- a polymer and a molecule with a low molecular weight (condensation)
The distinction between "step-growth polymerization" and "chain-growth polymerization" was instead introduced by Paul Flory in 1953, and are referred to the reaction mechanisms, respectively:[4]
- by functional groups (step-growth polymerization)
- by free-radical or ion (chain-growth polymerization)
The main characteristics are:
- polymerization process takes place in three distinct steps:
- chain initiation, usually by means of an initiator which starts the chemical process. Typical initiators include any organic compound with a labile group: e.g. azo (-N=N-), disulfide (-S-S-), or peroxide (-O-O-). Two examples are benzoyl peroxide and AIBN.
- chain propagation
- chain termination, which occurs either by combination or disproportionation. Termination, in radical polymerization, is when the free radicals combine and is the end of the polymerization process.
- some side reactions may occur, such as: chain transfer to monomer, chain transfer to solvent, and chain transfer to polymer.
- unlike condensation polymerization (also known as step-growth polymerization):
- high molecular weight polymer is formed at low conversion
- no small molecules, such as H2O, are eliminated in this process
- new monomer adds on the growing polymer chain via the reactive active centre which can be a
- free radical in radical polymerization
- carbocation in cationic polymerization
- carbanion in anionic polymerization
- organometallic complex in coordination polymerization.
- the monomer molecule can be a
- unsaturated compound like ethylene or acetylene which make them reactive, see vinyl polymer
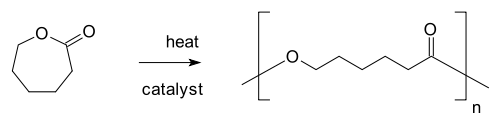
- Alicyclic compound, see ring-opening polymerization
- given special reactants and reaction conditions an addition polymerization can be considered a living polymerization.
- above a certain ceiling temperature, no polymerization occurs.
Examples
- Benzoyl peroxide is a radical initiator for the free radical addition polymerization of styrene to produce polystyrene.
- Aluminium chloride is an initiator for the cationic addition polymerization of isobutylene to form isobutyl synthetic rubber.
References
- ^ Introduction to Polymers 1987 R.J. Young Chapman & Hall ISBN 0-412-22170-5
- ^ W. H. Carothers (1929). "Studies On Polymerization And Ring Formation. I. An Introduction To The General Theory Of Condensation Polymers". Journal of American Chemical Society 51 (8): 2548–59. doi:10.1021/ja01383a041.
- ^ Paul J. Flory, "Principles of Polymer Chemistry", Cornell University Press, 1953, p.39. ISBN 0-8014-0134-8
- ^ Susan E. M. Selke, John D. Culter, Ruben J. Hernandez, "Plastics packaging: Properties, processing, applications, and regulations", Hanser, 2004, p.29. ISBN 1-56990-372-7
External links
Categories:- Polymer chemistry
- Polymerization reactions
Wikimedia Foundation. 2010.