- Aesculetin
-
Aesculetin[1] 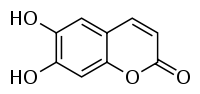 6,7-Dihydroxy-2-chromenoneOther namesEsculetin
6,7-Dihydroxy-2-chromenoneOther namesEsculetin
Cichorigenin
6,7-DihydroxycoumarinIdentifiers CAS number 305-01-1 
PubChem 5281416 ChemSpider 4444764 
KEGG C09263 
ChEBI CHEBI:490095 
ChEMBL CHEMBL244743 
Jmol-3D images Image 1
Image 2- C1=CC(=O)OC2=CC(=C(C=C21)O)O
O=C/2Oc1cc(O)c(O)cc1\C=C\2
Properties Molecular formula C9H6O4 Molar mass 178.14 g/mol Exact mass 178.026609  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Aesculetin (also known as esculetin, 6,7-dihydroxycoumarin and cichorigenin) is a derivative of coumarin. It is a natural lactone that derives from the intramolecular cyclization of a cinnamic acid derivative.
It is present in many toxic and medicinal plants, in form of glycosides and caffeic acid conjugates.[2]
Esculetin-containing preparations used systemically can have an anticoagulant effect and might interact with anticoagulent drugs such as warfarin.
This compound is used in some sunscreens, but there is evidence that it acts as a photosensitizer for DNA damage.[3] The sodium salt of its methyl-derivative is used in dermatology for the treatment of varicose veins.[4]
See also
- Aesculin, the glucoside of aesculetin
- Umbelliferone
References
- ^ Aesculetin at Sigma-Aldrich
- ^ Plant biochemistry (1997) P. M. Dey & Jeffrey B. Harborne (eds.)
- ^ Hausen BM, Schmieder M (September 1986). "The sensitizing capacity of coumarins (I)". Contact Dermatitis 15 (3): 157–63. doi:10.1111/j.1600-0536.1986.tb01317.x. PMID 3780217.
- ^ "Permethol" Data Sheet
Aglycones glycosides Aesculin | Skimmin (7-O-β-D-glucopyranosylumbelliferone) | ScopolinFuran derivatives Furanocoumarins (Angelicin | Apterin | Bergamottin | Bergapten | Imperatorin | Marmesin | Methoxsalen | Psoralen | Trioxsalen) | FuranochromonesMonoterpene coumarin ether Synthetic or drugs Categories:- Coumarins
- C1=CC(=O)OC2=CC(=C(C=C21)O)O
Wikimedia Foundation. 2010.
