- Chlorous acid
-
Chlorous acid 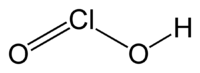
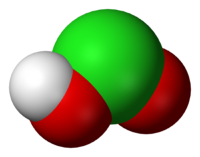 Chlorous acid
Chlorous acidIdentifiers CAS number 13898-47-0 
PubChem 24453 ChemSpider 22861 
KEGG C01486 
ChEBI CHEBI:29219 
Jmol-3D images Image 1 - O=ClO
Properties Molecular formula HClO2 Molar mass 68.46 g/mol Acidity (pKa) 1.96  acid (verify) (what is:
acid (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Chlorous acid is an inorganic compound with the formula HClO2. It is a weak acid. Chlorine has oxidation state +3 in this acid. The pure substance is unstable, disproportionating to hypochlorous acid (Cl oxidation state +1) and chloric acid (Cl oxidation state +5):
- 2 HClO2 → HClO + HClO3
Although the acid is difficult to obtain in pure substance, the conjugate base, chlorite, derived from this acid are stable. One example is the salt is well known sodium chlorite. This and related salts are sometimes used in the production of chlorine dioxide.
Preparation
HClO2 can be prepared through reaction of barium chlorite and dilute sulfuric acid:
- Ba(ClO2)2 + H2SO4 → BaSO4 + 2HClO2
Stability
Chlorous acid is a powerful oxidizing agent, although its tendency to disproportionation counteracts its oxidizing potential.
Chlorine is the only halogen to form an isolable acid of formula HXO2.[1] Fluorine resists oxidation above the 1+ level. Neither bromous acid nor iodous acid have been isolated. A few salts of bromous acid, bromites, are known, but no iodites.[1]
References
 Media related to Chlorous acid at Wikimedia Commons
Media related to Chlorous acid at Wikimedia Commons- ^ a b Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0-12-352651-5
Hydrogen compounds H3AsO3 · H3AsO4 · HAt · HSO3F · HBF4 · HBr · HBrO · HBrO2 · HBrO3 · HBrO4 · HCl · HClO · HClO2 · HClO3 · HClO4 · HCN · HCNO · H2CrO4/H2Cr2O7 · H2CO3 · H2CS3 · HF · HFO · HI · HIO · HNC · HNCO · HNO · HNO3 · H2N2O2 · HNO5S · H3NSO3 · H2O · H2O2 · H2O3 · H3PO2 · H3PO3 · H3PO4 · H4P2O7 · H5P3O10 · H2PtCl6 · H2S · H2Se · H2SeO3 · H2SeO4 · H4SiO4 · H2SiF6 · H2SO3 · H2SO4 · H2SO5 · H2S2O3 · H2S2O6 · H2S2O7 · H2S2O8 · CF3SO3H · H2Te · H2TeO3 · H6TeO6 · H4TiO4 · H2Po · H3VO4 · HCo(CO)4
Categories:- Hydrogen compounds
- Chlorites
- Oxidizing acids
- Oxidizing agents
- Inorganic compound stubs
Wikimedia Foundation. 2010.
