- Nitroxyl
-
Nitroxyl  AzanoneSystematic nameOxidanimine[citation needed]Other namesHydrogen oxonitrate(I)
AzanoneSystematic nameOxidanimine[citation needed]Other namesHydrogen oxonitrate(I)
Nitronous oxide
Nitrosyl hydrideIdentifiers CAS number 14332-28-6 PubChem 945 ChemSpider 920 
MeSH Nitroxyl ChEMBL CHEMBL1200689 
Jmol-3D images Image 1 - N=O
Properties Molecular formula HNO Molar mass 31.01 g mol−1 Exact mass 31.005813659 g mol-1 log P 0.74 Structure Coordination
geometryDigonal Molecular shape Dihedral Thermochemistry Standard molar
entropy So298220.91 J K-1 mol-1 Specific heat capacity, C 33.88 J K-1 mol-1 Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references Nitroxyl is the chemical compound HNO. It is well known in the gas phase [1]. In aqueous solution it acts as an acid with the conjugate base NO−, (pKa = 11.4).[2] NO− is the reduced form of nitric oxide (NO) and is isoelectronic with dioxygen. Nitroxyl can be formed as a reaction intermediate.
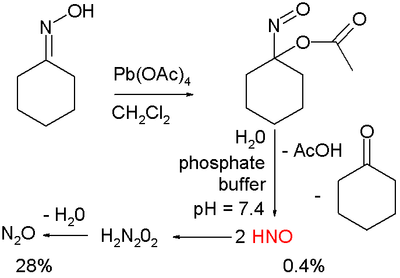
Nitroxyl is very reactive towards nucleophiles (especially thiols) and quickly dimerizes to hyponitrous acid, H2N2O2, which is then dehydrated to nitrous oxide N2O. Therefore, HNO is generally prepared in situ for example with the compounds such as Angeli’s salt (Na2N2O3) and Piloty’s acid (PhSO2NHOH) when it is needed.Nitroxyl shows potential in the treatment of heart failure and ongoing research is focused on finding new nitroxyl donors. In one study [3] such donor is prepared by organic oxidation of cyclohexanone oxime with lead tetraacetate to 1-nitrosocyclohexyl acetate:
This compound can be hydrolyzed under basic conditions in a phosphate buffer to nitroxyl HNO, acetic acid and cyclohexanone.
Other studies that have been conducted on HNO precursors include those from Nagasawa et al. [4] in which Piloty's acid is derivatized and produces HNO upon thermal decomposition. Other notable studies on the production of HNO come from Toscano et al.[5] in which cycloadducts of acyl nitroso species (which are known to decompose via hydrolysis to HNO and acyl acid) are synthesized. Upon photolysis these compounds release the acyl nitroso species which then further decomposes.
References
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 0080379419.
- ^ Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0-12-352651-5
- ^ Hydrolysis of Acyloxy Nitroso Compounds Yields Nitroxyl (HNO) Xin Sha, T. Scott Isbell, Rakesh P. Patel, Cynthia S. Day, and S. Bruce King J. Am. Chem. Soc.; 2006; 128(30) pp 9687 - 9692; (Article) doi: 10.1021/ja062365a
- ^ Prodrugs of Nitroxyl as Potential Aldehyde Dehydrogenase Inhibitors vis-a-vis Vascular Smooth Muscle Relaxants Nagasawa, H. T.; Kawle, S. P.; Elberling, J. A.; DeMaster, E. G.; Fukuto, J. M. J. Med. Chem., 38, 1865-1871. doi:10.1021/jm00011a005
- ^ Cohen, A. D.; Zeng, B.-B.; King, S. B.; Toscano, J. P. J. Am. Chem. Soc. 2003, 125, 1444-1445.doi:10.1021/ja028978e
Hydrogen compounds H3AsO3 · H3AsO4 · HAt · HSO3F · HBF4 · HBr · HBrO · HBrO2 · HBrO3 · HBrO4 · HCl · HClO · HClO2 · HClO3 · HClO4 · HCN · HCNO · H2CrO4/H2Cr2O7 · H2CO3 · H2CS3 · HF · HFO · HI · HIO · HNC · HNCO · HNO · HNO3 · H2N2O2 · HNO5S · H3NSO3 · H2O · H2O2 · H2O3 · H3PO2 · H3PO3 · H3PO4 · H4P2O7 · H5P3O10 · H2PtCl6 · H2S · H2Se · H2SeO3 · H2SeO4 · H4SiO4 · H2SiF6 · H2SO3 · H2SO4 · H2SO5 · H2S2O3 · H2S2O6 · H2S2O7 · H2S2O8 · CF3SO3H · H2Te · H2TeO3 · H6TeO6 · H4TiO4 · H2Po · H3VO4 · HCo(CO)4
Categories:- Hydrogen compounds
- Nitrogen oxoacids
- Oxygen compounds
Wikimedia Foundation. 2010.