- Non-Kekulé molecule
-
A non-Kekulé molecule is a conjugated hydrocarbon that cannot be assigned classical Kekulé structures. Since non-Kekulé molecules have two or more formal radical centers, their spin-spin interactions can cause electrical conductivity or ferromagnetism (molecule-based magnets), and applications to functional materials are expected. However, as these molecules are quite reactive and most of them are easily decomposed or polymerized at room temperature, strategies for stabilization are needed for their practical use. Synthesis and observation of these reactive molecules are generally accomplished by matrix-isolation methods. The simplest non-Kekulé molecules are biradicals.
Contents
Biradicals
A biradical in organic chemistry is an even-electron chemical compound with two free radical centres which act independently of each other and not to be confused with diradicals in general.[1]
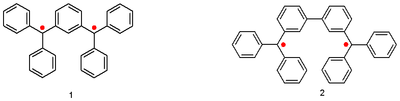
One of the first biradicals was synthesized by Wilhelm Schlenk in 1915 following the same methodology as Moses Gomberg's triphenylmethyl radical. The so-called Schlenk-Brauns hydrocarbons are:[2]
Eugene Müller, with the aid of a Gouy balance, established for the first time that these compounds are paramagnetic with a triplet ground state.
Other classic biradicals are those synthesed by Tschitschibabin [3] [4], Yang [5] and Coppinger [6] [7] [8].
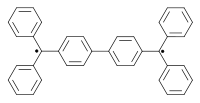
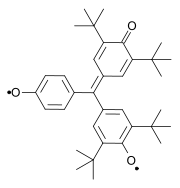
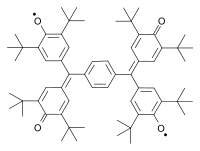
Tschitschibabin biradical (1907) Yang biradical (1960) Coppinger biradical 1962 Trimethylenemethane
A well studied biradical is trimethylenemethane or TMM:
This molecule can be obtained from photolysis of a diazo precursor with expulsion of nitrogen or from photolysis of 2-methylenecyclobutanone with expulsion of carbon monoxide. In 1966 Paul Dowd determined with electron spin resonance that this compound also has a triplet state. In a crystalline host the 6 hydrogen atoms in TMM are identical. Recombination of the two radicals to a cyclopropane full valence compound is only possible when the triplet state converts to the higher energy singlet state first.
The most common use of the TMM framework is in transition metal chemistry and specifically the use in trimethylenemethane cycloaddition reaction. In 1979, Trost et al. published a palladium catalyzed [3+2] cycloaddition of trimethylenemethane. [9]
Quinodimethanes & PAH's
Non-Kekulé quinodimethanes are biradicaloids of a six-membered ring with methylene substituents. Non-Kekulé polynuclear aromatics are composed of several fused six-membered rings. The synthesis of Triangulene, the simplest non-Kekulé polynuclear aromatic, was first tried by Eric Clar in 1953, but it was not even observed until the syntheses of trioxytriangulene by Richard J. Bushby in 1995 and kinetically stabilized triangulene by Kazuhiro Nakasuji in 2001. A related class of biradicals are para-benzynes.
Other studied biradicals are those based on Pleiadene [10], extended viologens [11] [12], corannulenes [13], nitronyl-nitroxide [14], bis(phenalenyl)s [15] and teranthenes [16] [17].

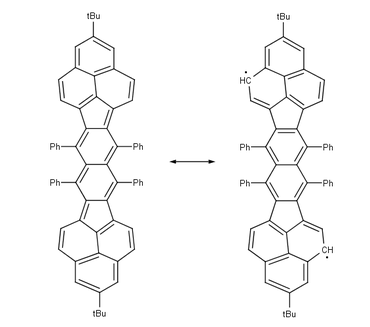
Teranthene biradical Singlet. max. 3 stabilizing Clar sextets, stable rt, air. 50% biradical, molecular section of graphene Bisphenalenyl biradical Singlet. max. 6 stabilizing Clar sextets, stable rt, air. 42% biradical Pleiadene has been synthesised from acenaphthylene and anthranilic acid / amyl nitrite:
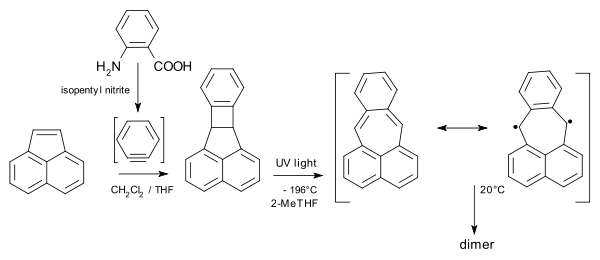
Pleiadene generation and dimerization Oxyallyl
In the oxyallyl diradical (OXA) one methylene group in TMM is replaced by oxygen. This reactive intermediate is postulated to occur in ring opening of cyclopropanones, allene oxides and in the Favorskii rearrangement. The intermediate has been produced by reaction of oxygen radical anions with acetone and studied by photoelectron spectroscopy [18]. The experimental electron affinity of OXA is 1.94 eV.
Classification
Non-Kekulé molecules with two formal radical centers (non-Kekulé diradicals) can be classified into non-disjoint and disjoint by the shape of their two non-bonding molecular orbitals (NBMOs).
Both NBMOs of molecules with non-disjoint characteristics such as trimethylenemethane (TMM) have electron density at the same atom. According to Hund's rule, each orbital is filled with one electron with parallel spin, avoiding the Coulomb repulsion by filling one orbital with two electrons. Therefore, such molecules with non-disjoint NBMOs are expected to prefer a triplet ground state.
In contrast, the NBMOs of the molecules with disjoint characteristics such as tetramethyleneethane (TME) can be described without having electron density at the same atom. With such MOs, the destabilization factor by the Coulomb repulsion becomes much smaller than with non-disjoint type molecules, and therefore the relative stability of the singlet ground state to the triplet ground state will be nearly equal, or even reversed because of exchange interaction.
References
- ^ IUPAC Gold Book definition biradicalPDF; diradicals
- ^ Reactive Intermediate Chemistry Robert A. Moss Ed., Wiley-Interscience 2004 ISBN 0471233242
- ^ Tschitschibabin, A. E. (1907), Über einige phenylierte Derivate des p, p-Ditolyls. Berichte der deutschen chemischen Gesellschaft, 40: 1810–1819. doi:10.1002/cber.19070400282
- ^ The molecular structures of Thiele's and Chichibabin's hydrocarbons Lawrence K. Montgomery, John C. Huffman, Edward A. Jurczak, Martin P. Grendze J. Am. Chem. Soc., 1986, 108 (19), pp 6004–6011 doi:10.1021/ja00279a056
- ^ SYNTHESIS OF A STABLE BIRADICAL N. C. Yang, A. J. Castro J. Am. Chem. Soc., 1960, 82 (23), p 6208 doi:10.1021/ja01508a067
- ^ A stable phenoxy radical inert to oxygen Tetrahedron, Volume 18, Issue 1, 1962, Pages 61-65 G. M. Coppinger doi:10.1016/0040-4020(62)80024-6
- ^ Inhibition Reactions of Hindered Phenols G. M. Coppinger J. Am. Chem. Soc., 1964, 86 (20), pp 4385–4388 doi:10.1021/ja01074a032
- ^ M. Baumgarten, "High spin molecules directed towards molecular magnets", in "EPR of free radicals in solids, Trends in methods and application", A. Lund, M. Shiotani (eds), Kluwer, 2003/2004, chapt. 12, pp. 491-528
- ^ New conjunctive reagents. 2-Acetoxymethyl-3-allyltrimethylsilane for methylenecyclopentane annulations catalyzed by palladium(0) Barry M. Trost, Dominic M. T. Chan J. Am. Chem. Soc., 1979, 101 (21), pp 6429–6432 doi:10.1021/ja00515a046
- ^ .pi.,.pi.-Biradicaloid hydrocarbons. Pleiadene family. I. Photochemical preparation from cyclobutene precursors Jaroslav. Kolc, Josef. Michl J. Am. Chem. Soc., 1973, 95 (22), pp 7391–7401 doi:10.1021/ja00803a030
- ^ Synthesis and Characterization of a Highly Reducing Neutral “Extended Viologen” and the Isostructural Hydrocarbon 4,4‘‘‘‘-Di-n-octyl-p-quaterphenyl William W. Porter III, Thomas P. Vaid, and Arnold L. Rheingold J. Am. Chem. Soc., 2005, 127 (47), pp 16559–16566 doi:10.1021/ja053084q
- ^ Casado, J., Patchkovskii, S., Zgierski, M., Hermosilla, L., Sieiro, C., Moreno Oliva, M. and López Navarrete, J. (2008), Raman Detection of “Ambiguous” Conjugated Biradicals: Rapid Thermal Singlet-to-Triplet Intersystem Crossing in an Extended Viologen. Angewandte Chemie International Edition, 47: 1443–1446. doi:10.1002/anie.200704398
- ^ Ueda, A., Nishida, S., Fukui, K., Ise, T., Shiomi, D., Sato, K., Takui, T., Nakasuji, K. and Morita , Y. (2010), Three-Dimensional Intramolecular Exchange Interaction in a Curved and Nonalternant π-Conjugated System: Corannulene with Two Phenoxyl Radicals. Angewandte Chemie International Edition, 49: 1678–1682. doi:10.1002/anie.200906666 PMID 20108294
- ^ Strong Exchange Interactions between Two Radicals Attached to Nonaromatic Spacers Deduced from Magnetic, EPR, NMR, and Electron Density Measurements Raymond Ziessel Christophe Stroh, Henrike Heise, Frank H. Köhler, Philippe Turek,Nicolas Claiser, Mohamed Souhassou, and Claude Lecomte J. Am. Chem. Soc., 2004, 126 (39), pp 12604–12613 doi:10.1021/ja0305959
- ^ Singlet Biradical Character of Phenalenyl-Based Kekulé Hydrocarbon with Naphthoquinoid Structure Takashi Kubo, Akihiro Shimizu, Mikio Uruichi, Kyuya Yakushi, Masayoshi Nakano, Daisuke Shiomi, Kazunobu Sato, Takeji Takui, Yasushi Morita, Kazuhiro Nakasuji Org. Lett., 2007, 9 (1), pp 81–84 doi:10.1021/ol062604z
- ^ Synthesis and Characterization of Teranthene: A Singlet Biradical Polycyclic Aromatic Hydrocarbon Having Kekule Structures Akihito Konishi, Yasukazu Hirao, Masayoshi Nakano, Akihiro Shimizu, Edith Botek, Benot Champagne, Daisuke Shiomi, Kazunobu Sato, Takeji Takui, Kouzou Matsumoto, Hiroyuki Kurata and Takashi Kubo J. Am. Chem. Soc., 2010, 132 (32), pp 11021–11023 doi:10.1021/ja1049737
- ^ Lambert, C. (2011), Towards Polycyclic Aromatic Hydrocarbons with a Singlet Open-Shell Ground State. Angewandte Chemie International Edition, 50: 1756–1758. doi:10.1002/anie.201006705
- ^ The Lowest Singlet and Triplet States of the Oxyallyl Diradical Takatoshi Ichino, Stephanie M. Villano, Adam J. Gianola, Daniel J. Goebbert, Luis Velarde, Andrei Sanov, Stephen J. Blanksby, Xin Zhou, David A. Hrovat, Weston Thatcher Borden, and W. Carl Lineberger Angew. Chem. Int. Ed. 2009, 48, 8509 –8511 doi:10.1002/anie.200904417
Categories:- Organic chemistry
- Free radicals
Wikimedia Foundation. 2010.