- Coumaric acid
-
p-Coumaric acid 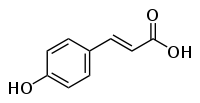
 3-(4-hydroxyphenyl)-2-propenoic acidOther namespara-coumaric acid,
3-(4-hydroxyphenyl)-2-propenoic acidOther namespara-coumaric acid,4-hydroxycinnamic acid,
β-(4-hydroxyphenyl)acrylic acidIdentifiers CAS number 501-98-4 
PubChem 637542 ChemSpider 553148 
EC number 231-000-0 DrugBank DB04066 ChEBI CHEBI:32374 
ChEMBL CHEMBL66879 
Jmol-3D images Image 1
Image 2- C1=CC(=CC=C1\C=C\C(=O)O)O
c1cc(ccc1/C=C/C(=O)O)O
Properties Molecular formula C9H8O3 Molar mass 164.16 g mol−1 Exact mass 164.047344 Melting point 210–213 °C
Hazards R-phrases R36/37/38 S-phrases S24/25  acid (verify) (what is:
acid (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Coumaric acid is a hydroxycinnamic acid, an organic compound that is a hydroxy derivative of cinnamic acid. There are three isomers, o-coumaric acid, m-coumaric acid, and p-coumaric acid, that differ by the position of the hydroxy substitution of the phenyl group. p-Coumaric acid is the most abundant isomer of the three in nature.
Together with sinapyl alcohol and coniferyl alcohols, p-coumaric acid is a major component of lignocellulose. It is biosynthesized from cinnamic acid by the action of the P450-dependent enzyme 4-cinnamic acid hydroxylase.
p-Coumaric acid can be found in a wide variety of edible plants such as peanuts, tomatoes, carrots, and garlic. It is a crystalline solid that is slightly soluble in water, but well soluble in ethanol and diethyl ether.
p-Coumaric acid has antioxidant properties and is believed to reduce the risk of stomach cancer[1] by reducing the formation of carcinogenic nitrosamines.[2]
Biochemistry
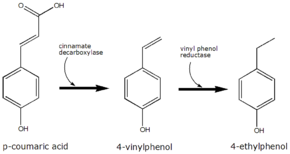
p-Coumaric acid is the precursor of 4-ethylphenol produced by the yeast Brettanomyces in wine. The yeast converts this to 4-vinylphenol via the enzyme cinnamate decarboxylase.[3] 4-Vinylphenol is further reduced to 4-ethylphenol by the enzyme vinyl phenol reductase. Coumaric acid is sometimes added to microbiological media, enabling the positive identification of Brettanomyces by smell.
See also
- Coumaroyl-Coenzyme A
- Ferulic acid
- Cinnamic acid
References
- ^ Ferguson LR, Shuo-tun Z, Harris PJ (2005). "Antioxidant and antigenotoxic effects of plant cell wall hydroxycinnamic acids in cultured HT-29". Molecular Nutrition & Food Research 49 (6): 585–693. doi:10.1002/mnfr.200500014. PMID 15841493.
- ^ Kikugawa K, Hakamada T, Hasunuma M, Kurechi T (1983). "Reaction of p-hydroxycinnamic acid derivatives with nitrite and its relevance to nitrosamine formation". Journal of Agricultural and Food Chemistry 1 (4): 780–785. doi:10.1021/jf00118a025.
- ^ Brettanomyces Monitoring by Analysis of 4-ethylphenol and 4-ethylguaiacol at etslabs.com
Hydroxycinnamic acids Caffeic acid | Cichoric acid | Cinnamic acid | Coumaric acid | Diferulic acid | Ferulic acid | Plicatins A and B | Sinapinic acidGlycosides Tartaric acid esters Caftaric acid | Coutaric acid | Fertaric acidOthers Grape reaction productCategories:- Hydroxycinnamic acids
- Phenolic compounds in wine
- C1=CC(=CC=C1\C=C\C(=O)O)O
Wikimedia Foundation. 2010.