- Nitrosyl fluoride
-
Nitrosyl fluoride 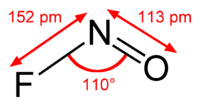
 Nitrosyl fluorideOther namesNOF
Nitrosyl fluorideOther namesNOF
Nitrogen oxyfluorideIdentifiers CAS number 7789-25-5 PubChem 123261 ChemSpider 109874 
Jmol-3D images Image 1 - FN=O
Properties Molecular formula NOF Molar mass 49.0045 g/mol Appearance colourless gas
bluish with impuritiesDensity 1.719 g/cm3 (solid)
1.326 g/mL (liquid)
2.657 g/L (gas)Melting point -166°C (107.15 K)
Boiling point -72.4°C (200.75 K)
Solubility in water Reacts. Related compounds Other anions nitrosyl chloride
nitrosyl bromideOther cations nitryl fluoride, thionyl fluoride  fluoride (verify) (what is:
fluoride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Nitrosyl fluoride, NOF, is a covalently bonded nitrosyl compound.
Contents
Reactions
NOF is a highly reactive fluorinating agent that converts many metals to their fluorides, releasing nitric oxide:
- n NOF + M → MFn + n NO
NOF also fluorinates fluorides to form adducts that have a salt-like character, such as NOBF4.
Aqueous solutions of NOF are powerful solvents for metals, by a mechanism similar to that seen in aqua regia. Nitrosyl fluoride reacts with water to form nitrous acid, which then forms nitric acid:
- NOF + H2O → HNO2 + HF
- 3 HNO2 → HNO3 + 2 NO + H2O
Nitrosyl fluoride can also convert alcohols to nitrites:
- ROH + NOF → RONO + HF
Uses
Nitrosyl fluoride is used as a solvent[citation needed] and as a fluorinating and nitrating agent in organic synthesis.[citation needed] It is also used as an oxidizer in rocket propellants.
References
Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 0080379419.
External links
Categories:- Nitrosyl compounds
- Oxofluorides
- Fluorinating agents
Wikimedia Foundation. 2010.
