- Halogenation
-
- "Fluorination" redirects here. For the addition of fluoride to drinking water, see water fluoridation.
Halogenation is a chemical reaction that incorporates a halogen atom into a molecule in substitution of hydrogen atom. Halogenation takes place in the gas phase. There are four types of halogenation: fluorination, chlorination, bromination, and iodination. There are several reaction mechanisms of halogenation including free radical halogenation, ketone halogenation, electrophilic halogenation, and halogen addition reaction.
Dehalogenation is removal of halogen from a molecule.
Contents
Reaction types
The site of halogenation is significantly determined by the size and reactivity of the halogen. Fluorine and chlorine are more electronegative and have smaller atomic weight. Fluorination and chlorination are more likely to form meso compounds.[1] Bromine and iodine are larger and less reactive. Bromination and iodination are more likely to substitute at the beta carbon.[1]
In an endothermic reaction, the transition state is close to the products in energy and structure. In an exothermic reaction, the transition state is close to the reactants in energy and in structure.
Fluorination
Fluorine reacts explosively with methane.
Chlorination
Chlorine reacts at a moderate rate with methane.
Chlorination is a reaction that goes to completion because its equilibrium constant is so large. Chlorination is generally highly exothermic and, as in most organic reactions, this decrease in enthalpy is a primary driving force. The highest point in the energy diagram of the chlorination of methane is the transition state for the reaction of methane with a chlorine radical.
Bromination
Bromine needs heat to react with methane.
Bromination is more selective than chlorination because the major reaction is favored by a larger amount. This is because of bromination’s larger activation energy compared to chlorination. The energy differences between chlorination and bromination result from the difference in the bond dissociation energy of H-Cl and H-Br. H-Br is weaker, and hydrogen abstraction from Br is endothermic. The first propagation is endothermic for bromination but exothermic for chlorination.
The transition states forming the 1o and 2o radicals for the endothermic bromination have larger energy difference than those for the exothermic chlorination, even though the energy difference of the products is the same (13 kJ, or 3 kcal).
Iodination
Iodine does not react at all with methane.
Although porphyrin can undergo any type of halogenation, iodination of porphyrin is least favored because of steric factors and electronic effect.[1]
Reaction mechanisms
In a Markovnikov addition reaction, a halogen reacts with an alkene causing the π-bond to break forming a haloalkane. This makes the hydrocarbon more reactive and bromine as it turns out, is a good leaving group in further chemical reactions such as nucleophilic aliphatic substitution reactions and elimination reactions.
Halogenation is a substitution. Halogen atom replaces a hydrogen.
In higher alkanes, replacement of different hydrogen atoms leads to different products. The preference for reaction at the secondary position results from greater stability of the secondary free radical and the transition state leading to it. The bond-dissociation enthalpy to form a free radical by a breaking a bond between a hydrogen atom and a carbon atom is greatest for a methyl carbon, and it decreases for a primary carbon, a secondary carbon, and a tertiary carbon. The more highly substituted the carbon, the less energy is required to form the free radical. Free radicals are more stable if they are more highly substituted.
Examples
The formation of gold(III) chloride by the chlorination of gold.
Specific halogenation methods are the Hunsdiecker reaction (from carboxylic acids) and the Sandmeyer reaction (arylhalides).
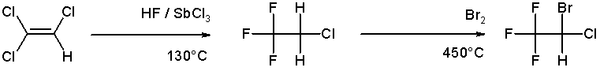
An example of halogenation can be found in the organic synthesis of the anesthetic halothane from trichloroethylene which involves a high temperature bromination in the second step [2]:
See also
- Haloalkane (Alkyl halide)
- Halogenoarene (Aryl halide)
- Free radical halogenation
- Haloketone
- Electrophilic substitution
References
- ^ a b c ISBN=0123932017
- ^ Synthesis of essential drugs, Ruben Vardanyan, Victor Hruby; Elsevier 2005 ISBN 0-444-52166-6
Categories:- Organic reactions
- Inorganic reactions
- Halogens
Wikimedia Foundation. 2010.