- Dithiothreitol
-
Dithiothreitol[1]  (2S,3S)-1,4-bis(sulfanyl)butane-2,3-diolOther names(2S,3S)-1,4-dimercaptobutane-2,3-diol
(2S,3S)-1,4-bis(sulfanyl)butane-2,3-diolOther names(2S,3S)-1,4-dimercaptobutane-2,3-diol
D-threo-1,4-dimercaptobutane-2,3-diol
D-threo-1,4-dimercapto-2,3-butanediol
1,4-dithio-D-threitol
Cleland's reagent
ReductacrylIdentifiers CAS number 3483-12-3 
PubChem 446094 ChemSpider 393541 
UNII T8ID5YZU6Y 
DrugBank DB04447 ChEBI CHEBI:42170 
ChEMBL CHEMBL406270 
Jmol-3D images Image 1 - C([C@H]([C@@H](CS)O)O)S
Properties Molecular formula C4H10O2S2 Molar mass 154.25 g mol−1 Appearance White solid Melting point 42-43 °C
Boiling point 125-130 °C at 2 mmHg
Solubility in water Soluble  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
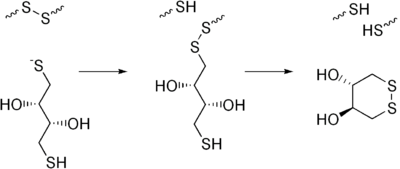
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dithiothreitol (DTT) is the common name for a small-molecule redox reagent known as Cleland's reagent. DTT's formula is C4H10O2S2 and the molecular structure of its reduced form is shown at the right; its oxidized form is a disulfide-bonded 6-membered ring (shown below). Its name derives from the four-carbon sugar, threose. DTT has an epimeric ('sister') compound, dithioerythritol (DTE).
Contents
Reducing agent
DTT is an unusually strong reducing agent, owing to its high conformational propensity to form a six-membered ring with an internal disulfide bond. It has a redox potential of -0.33 V at pH 7. The reduction of a typical disulfide bond proceeds by two sequential thiol-disulfide exchange reactions and is illustrated below. The intermediate mixed-disulfide state is unstable (i.e., poorly populated) because the second thiol of DTT has a high propensity to close the ring, forming oxidized DTT and leaving behind a reduced disulfide bond. The reducing power of DTT is limited to pH values above ~7, since only the negatively charged thiolate form -S– is reactive (the protonated thiol form -SH is not); the pKa of the thiol groups is 9.2 and 10.1.
Applications
A common use of DTT is as a reducing or "deprotecting" agent for thiolated DNA. The terminal sulfur atoms of thiolated DNA have a tendency to form dimers in solution, especially in the presence of oxygen. Dimerization greatly lowers the efficiency of subsequent coupling reactions such as DNA immobilization on gold in biosensors. Typically DTT is mixed with a DNA solution and allowed to react, and then is removed by filtration (for the solid catalyst) or by chromatography (for the liquid form). The DTT removal procedure is often called "desalting."
DTT is frequently used to reduce the disulfide bonds of proteins and, more generally, to prevent intramolecular and intermolecular disulfide bonds from forming between cysteine residues of proteins. However, even DTT cannot reduce buried (solvent-inaccessible) disulfide bonds, so reduction of disulfide bonds is sometimes carried out under denaturing conditions (e.g., at high temperatures, or in the presence of a strong denaturant such as 6 M guanidinium hydrochloride, 8 M urea, or 1% sodium dodecylsulfate). Conversely, the solvent exposure of different disulfide bonds can be assayed by their rate of reduction in the presence of DTT.
DTT can also be used as an oxidizing agent. Its principal advantage is that effectively no mixed-disulfide species are populated, in contrast to other agents such as glutathione. In very rare cases, a DTT adduct may be formed, i.e., the two sulfur atoms of DTT may form disulfide bonds to different sulfur atoms; in such cases, DTT cannot cyclize since it has no remaining free thiols.
Properties
Due to air oxidation, DTT is a relatively unstable compound whose useful life can be extended by refrigeration and handling in an inert atmosphere. Since protonated sulfurs have lowered nucleophilicities, DTT becomes less potent as the pH lowers. Tris(2-carboxyethyl)phosphine HCl (TCEP hydrochloride) is an alternative which is more stable and works even at low pH.
References
- ^ Merck Index, 11th Edition, 3382.
- Cleland, W.W. (April 1964). "Dithiothreitol, A New Protective Reagent for SH Groups". Biochemistry 3: 480–2. doi:10.1021/bi00892a002. PMID 14192894.
- Ruegg, UT; Rudinger, J. (1977). "Cleavage of disulfide bonds in proteins". Methods Enzymol 47: 111.
Categories:- Thiols
- Reducing agents
- Diols
Wikimedia Foundation. 2010.