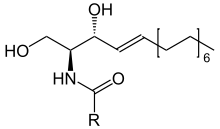
- Ceramide
-
 General chemical structure of sphingolipids. When the substituent (R) in this structure is H, the resulting molecule is a ceramide.
General chemical structure of sphingolipids. When the substituent (R) in this structure is H, the resulting molecule is a ceramide.
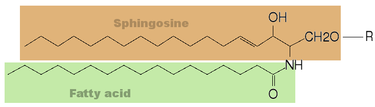
Ceramides (pronounced ser-A-mid) are a family of lipid molecules. A ceramide is composed of sphingosine and a fatty acid. Ceramides are found in high concentrations within the cell membrane of cells. They are one of the component lipids that make up sphingomyelin, one of the major lipids in the lipid bilayer. For years, it was assumed that ceramides and other sphingolipids found in the bilayer cell membrane were purely structural elements. This is now known to be not completely true. Ceramide can actually act as a signaling molecule. The most well-known functions of ceramides as cellular signals include regulating the differentiation, proliferation, programmed cell death (PCD), and apoptosis (Type I PCD) of cells.
Contents
Pathways for ceramide synthesis
There are three major pathways of ceramide generation. The sphingomyelinase pathway uses an enzyme to breakdown sphingomyelin in the cell membrane and release ceramide. The de novo pathway creates ceramide from less complex molecules. Ceramide generation can also occur through breakdown of complex sphingolipids that are ultimately broken down into sphingosine, which is then reused by reacylation to form ceramide. This latter pathway is termed the Salvage pathway.
Sphingomyelin hydrolysis
Hydrolysis of sphingomyelin is catalyzed by the enzyme sphingomyelinase. Because sphingomyelin is one of the four common phospholipids found in the plasma membrane of cells, the implications of this method of generating ceramide is that the cellular membrane is the target of extracellular signals leading to programmed cell death. There has been research suggesting that when ionizing radiation causes apoptosis in some cells, the radiation leads to the activation of sphingomyelinase in the cell membrane and ultimately, to ceramide generation.[1]
De novo
De novo synthesis of ceramide begins with the condensation of palmitate and serine to form 3-keto-dihydrosphingosine. This reaction is catalyzed by the enzyme serine palmitoyl transferase and is the rate-limiting step of the pathway. In turn, 3-keto-dihydrosphingosine is reduced to dihydrosphingosine, which is then followed by acylation by the enzyme (dihydro)ceramide synthase to produce dihydroceramide. The final reaction to produce ceramide is catalyzed by dihydroceramide desaturase. De novo synthesis of ceramide occurs in the endoplasmic reticulum. Ceramide is subsequently transported to the Golgi by either vesicular trafficking or the ceramide transfer protein CERT. Once in the Golgi apparatus, ceramide can be further metabolized to other sphingolipids, such as sphingomyelin and the complex glycosphingolipids.[2]
The salvage pathway
Constitutive degradation of sphingolipids and glycosphingolipids takes place in the acidic subcellular compartments, the late endosomes and the lysosomes. In case of glycosphingolipids, exohydrolases, acting at acidic pH optima, cause the stepwise release of monosaccharide units from the end of the oligosaccharide chains one after the other leading to the generation of ceramide whereas sphingomyelin is converted to ceramide by acid sphingomyelinase. Ceramide can be further hydrolyzed by acid ceramidase to form sphingosine and a free fatty acid, both of which are able to leave the lysosome in contrast to ceramide. The long-chain sphingoid bases released from the lysosome may then re-enter pathways for synthesis of ceramide and/or sphingosine-1-phosphate. The salvage pathway re-utilizes long-chain sphingoid bases to form ceramide through the action of ceramide synthase. Thus, ceramide synthase family members probably trap free sphingosine released from the lysosome at the surface of the endoplasmic reticulum or in endoplasmic reticulum-associated membranes. It should also be noted that the salvage pathway has been estimated to contribute from 50% to 90% of sphingolipid biosynthesis [3]
Physiological roles of ceramide
As a bioactive lipid, ceramide has been implicated in a variety of physiological functions including apoptosis, cell growth arrest, differentiation, cell senescence, cell migration and adhesion.[2] Roles for ceramide and its downstream metabolites have also been suggested in a number of pathological states including cancer, neurodegeneration, diabetes, microbial pathogenesis, obesity, and inflammation.[4][5]
Apoptosis
One of the most studied roles of ceramide pertains to its function as a proapoptotic molecule. Apoptosis, a form of programmed cell death, is essential for the maintenance of normal cellular homeostasis and is an important physiological response to many forms of cellular stress. Ceramide accumulation has been found following treatment of cells with a number of apoptotic agents including ionizing radiation [1][6], UV light [7], TNF-alpha [8], and chemotherapeutic agents. This suggests a role for ceramide in the biological responses of all these agents. Because of its apoptosis-inducing effects in cancer cells, ceramide has been termed the “tumor suppressor lipid” . Several studies have attempted to define further the specific role of ceramide in the events of cell death and some evidence suggests ceramide functions upstream of the mitochondria in inducing apoptosis. However, owing to the conflicting and variable nature of studies into the role of ceramide in apoptosis, the mechanism by which this lipid regulates apoptosis remains elusive.[9].
Substances known to induce ceramide generation
- Anandamide
- Tetrahydrocannabinol and other Cannabinoids [10][11]
- TNF-alpha
- Fas ligand
- Endotoxin
- Chemotherapeutic agents
- 1,25 dihydroxy vitamin D
- gamma interferon
- heat
- ionizing radiation [1][12]
- Ceramidase Inhibitors
It is interesting to note that the substances that can cause ceramide to be generated tend to be stress signals that can cause the cells to go into programmed cell death. Ceramide thus acts as an intermediary signal that connects the external signal to the internal metabolism of the cells.
Mechanism by which ceramide signalling occurs
Currently, the means by which ceramide acts as a signaling molecule are not clear.
One hypothesis is that ceramide generated in the plasma membrane stabilizes smaller lipid platforms known as lipid rafts, allowing them to serve as platforms for signalling molecules. Moreover, as rafts can cross the entire lipid bilayer, they can serve as the link between signals outside of the cell to signals to be generated within the cell.
Ceramide has also been shown to form organized large channels traversing the mitochondrial outer membrane. This leads to the egress of proteins from the intermembrane space.[13][14][15]
References
- ^ a b c Haimovitz-Friedman A, Kan CC, Ehleiter D et al. (1994). "Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis". J. Exp. Med. 180 (2): 525–35. doi:10.1084/jem.180.2.525. PMC 2191598. PMID 8046331. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2191598.
- ^ a b Hannun, Y.A. and Obeid, L.M. (2008). "Principles of bioactive lipid signalling: lessons from sphingolipids". Nature Reviews: Molecular Cell Biology 9 (2): 139–150. doi:10.1038/nrm2329. PMID 18216770.
- ^ Kitatani, K., Idkowiak-Baldys, J., and Hannun, Y.A. (2008). "The sphingolipid salvage pathway in ceramide metabolism and signaling". Cell Signaling 30 (6): 1010–1018. doi:10.1016/j.cellsig.2007.12.006. PMC 2422835. PMID 18191382. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2422835.
- ^ Zeidan, Y.H., and Hannun, Y.A. (2007). "Translational aspects of sphingolipid metabolism". Trends Mol. Med. 13 (8): 327–336. doi:10.1016/j.molmed.2007.06.002. PMID 17588815.
- ^ Wu D, Ren Z, Pae M, Guo W, Cui X, Merrill AH, Meydani SN (2007). "Aging up-regulates expression of inflammatory mediators in mouse adipose tissue". The Journal of Immunology 179 (7): 4829–39. PMID 17878382. http://www.jimmunol.org/cgi/content/full/179/7/4829.
- ^ Dbaibo, G.S., Pushkareva, M.Y., Rachid, R.A., Alter, N., Smyth, M.J., Obeid, L.M., and Hannun, Y.A. (1998). "p53-dependent ceramide response to genotoxic stress". J. Clin. Invest. 102 (2): 329–339. doi:10.1172/JCI1180. PMC 508891. PMID 9664074. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=508891.
- ^ Rotolo, J.A., Zhang, J., Donipui, M., Lee, H., Fuks, Z., and Kolesnick, R.N. (2005). "Caspase-dependent and -independent activation of acid sphingomyelinase signaling". J. Biol. Chem. 280 (28): 26425–34. doi:10.1074/jbc.M414569200. PMID 15849201.
- ^ Dbaibo, G.S., El-Assad, W., Krikorian, A., Liu, B., Diab, K., Idriss, N.Z., El-Sabban, M., Driscoll, T.A., Perry, D.K., and Hannun, Y.A. (2001). "Ceramide generation by two distinct pathways in tumor necrosis factor alpha-induced cell death". FEBS Letters 503 (1): 7–12. doi:10.1016/S0014-5793(01)02625-4. PMID 11513845.
- ^ Taha, T.A., Mullen, T.D. and Obeid, L.M. (2006). "A house divided: ceramide, sphingosine, and sphingosine-1-phosphate in programmed cell death". Biochim. Biophys. Acta 1758 (12): 2027–36. doi:10.1016/j.bbamem.2006.10.018. PMC 1766198. PMID 17161984. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1766198.
- ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedVelasco; see Help:Cite errors/Cite error references no text - ^ http://www.ncbi.nlm.nih.gov/pubmed/15958274
- ^ Hallahan DE (1996). "Radiation-mediated gene expression in the pathogenesis of the clinical radiation response". Sem. Radiat. Oncol. 6 (4): 250–267. doi:10.1016/S1053-4296(96)80021-X. PMID 10717183.
- ^ Siskind LJ, Kolesnick RN, Colombini M (2002). "Ceramide Channels Increase the Permeability of the Mitochondrial Outer Membrane to Small Proteins". J. Biol. Chem. 277 (30): 26796–803. doi:10.1074/jbc.M200754200. PMC 2246046. PMID 12006562. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2246046.
- ^ Stiban J, Fistere D, Colombini M (2006). "Dihydroceramide hinders ceramide channel formation: Implications on apoptosis". Apoptosis 11 (5): 773–80. doi:10.1007/s10495-006-5882-8. PMID 16532372.
- ^ Siskind LJ, Kolesnick RN, Colombini M (2006). "Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations". Mitochondrion 6 (3): 118–25. doi:10.1016/j.mito.2006.03.002. PMC 2246045. PMID 16713754. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2246045.
External links
Glycoconjugates, lipids and glycolipids: sphingolipids and glycosphingolipids, and metabolic intermediates Ceramide Ganglioside
pathwayFrom gangliosideFrom globosideGlobotriaosylceramideFrom sphingomyelinFrom sulfatideTo sphingosineCeramideOther biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iCategories:- Lipids
Wikimedia Foundation. 2010.