- Ionone
-
Ionones 

 α: (3E)-4-(2,6,6-Trimethylcyclohex-2-en-1-yl)but-3-en-2-one
α: (3E)-4-(2,6,6-Trimethylcyclohex-2-en-1-yl)but-3-en-2-one
β: (3E)-4-(2,6,6-Trimethylcyclohex-1-en-1-yl)but-3-en-2-one
γ: (3E)-4-(2,2-Dimethyl-6-methylenecyclohexyl)but-3-en-2-oneOther namesCyclocitrylideneacetone, irisone, jononProperties Molecular formula C13H20O Molar mass 192.30 g/mol Density α: 0.933 g/cm3
β: 0.945 g/cm3Melting point β: -49 °C
Boiling point β: 126-128 °C at 12 mmHg
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references The ionones are a series of closely related chemical substances that are part of a group of compounds known as rose ketones, which also includes damascones and damascenones. Ionones are aroma compounds found in a variety of essential oils, including rose oil. beta-Ionone is a significant contributor to the aroma of roses, despite its relatively low concentration, and is an important fragrance chemical used in perfumery.[1] The ionones are derived from the degradation of carotenoids.
The carotenes alpha-carotene, beta-carotene, gamma-carotene, and the xanthophyll beta-cryptoxanthin, all contain beta-ionone, and thus have vitamin A activity because they can be converted by plant-eating animals to retinol and retinal. Carotenoids that do not contain the beta-ionone moiety cannot be converted to retinol, and thus have no vitamin A activity.
Biosynthesis
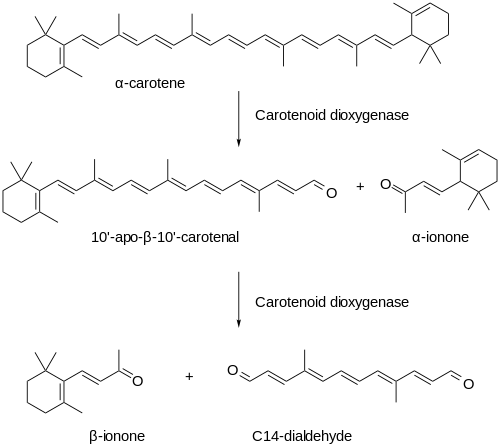
Carotenoids are the precursors of important fragrance compounds in several flowers. For example, a recent study of ionones in Osmanthus fragrans Lour. var. aurantiacus, exhibit the highest diversity of carotenoid-derived volatiles among the flowering plants investigated. A cDNA encoding a carotenoid cleavage enzyme, OfCCD1, was identified from transcripts isolated from flowers of O. fragrans Lour. It was found that the recombinant enzymes cleaved carotenes to produce α-ionone and β-ionone in in vitro assays.
It was also found in the same study that carotenoid content, volatile emissions, and OfCCD1 transcript levels are subject to photorhythmic changes, and principally increased during daylight hours. At the times when OfCCD1 transcript levels reached their maxima, the carotenoid content remained low or slightly decreased. The emission of ionones was also higher during the day; however, emissions decreased at a lower rate than the transcript levels. Moreover, carotenoid content increased from the first to the second day, whereas the volatile release decreased, and the OfCCD1 transcript levels displayed steady-state oscillations, suggesting that the substrate availability in the cellular compartments is changing or other regulatory factors are involved in volatile norisoprenoid formation. The formation of ionones proceeds by a process mediated by the carotenoid dioxygenases.[2]
Organic synthesis
Ionone can be synthesised from citral and acetone with calcium oxide as a basic heterogeneous catalysis and serves as an example of an aldol condensation followed by a rearrangement reaction.[3][4]
The nucleophilic addition of the carbanion 3 of acetone 1 to the carbonylgroup on citral 4 is base catalysed. The aldol condensation product 5 eliminates water through the enolate ion 6 to the pseudoionone 7.

The reaction proceeds by acid catalysis where the double bond in 7 opens to form the carbocation 8. A rearrangement reaction of the carbocation follows with ring closure to 9. Finally a hydrogen atom can be abstracted from 9 by an acceptor molecule (Y) to form either 10 (extended conjugated system) or 11.

References
- ^ Rose (Rosa damascena), John C. Leffingwell
- ^ Susanne Baldermann, Masaya Kato, Miwako Kurosawa, Yoshiko Kurobayashi, Akira Fujita, Peter Fleischmann, Naoharu Watanabe (2010). "Functional characterization of a carotenoid cleavage dioxygenase 1 and its relation to the carotenoid accumulation and volatile emission during the floral development of Osmanthus fragrans Lour". Journal of Experimental Botany 61 (11): 2967–2977. doi:10.1093/jxb/erq123. PMID 20478967.
- ^ NODA, C., ALT, G. P., WERNECK, R. M. et al. (1998). "Aldol Condensation of Citral with Acetone on Basic Solid Catalysts". Braz. J. Chem. Eng. 15 (2). doi:10.1590/S0104-66321998000200004.
- ^ Alfred Russell and R. L. Kenyon. "Pseudoionone". Organic Syntheses, Coll. Vol. 3 23: 78. http://www.orgsyn.org/orgsyn/default.asp?dbname=orgsyn&dataaction=search&metadata_directive=blind_gui&formgroup=quick_form_group&Preps.CollVol=3&Preps.CollPage=747&order_by=Preps.CollPage%20ASC.
Categories:- Perfume ingredients
- Flavors
- Ketones
- Terpenes and terpenoids
Wikimedia Foundation. 2010.