- Methyl isocyanate
-
Methyl isocyanate 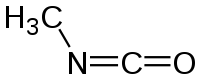
 Methyl isocyanateOther namesisocyanatomethane, methyl carbylamine
Methyl isocyanateOther namesisocyanatomethane, methyl carbylamineIdentifiers CAS number 624-83-9 
PubChem 12228 ChemSpider 11727 
ChEBI CHEBI:59059 
Jmol-3D images Image 1 - O=C=N/C
Properties Molecular formula C2H3NO Molar mass 57.051 g/mol Density 0.9230 g/cm3 at 27°C Melting point -45°C
Boiling point 39.5°C
Solubility in water very soluble[1] Vapor pressure 57.7 kPa Structure Dipole moment 2.8 D Thermochemistry Std enthalpy of
formation ΔfHo298-92.0 kJ·mol-1[1] Hazards NFPA 704 Flash point -7°C Autoignition
temperature534°C Explosive limits 5.3 - 26%[1] Related compounds Related compounds Methyl isothiocyanate  isocyanate (verify) (what is:
isocyanate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Methyl isocyanate (MIC) is an organic compound with the molecular formula CH3NCO. Synonyms are isocyanatomethane, methyl carbylamine, and MIC. Methyl isocyanate is an intermediate chemical in the production of carbamate pesticides (such as carbaryl, carbofuran, methomyl, and aldicarb). It has also been used in the production of rubbers and adhesives. As a highly toxic and irritating material, it is hazardous to human health, and was involved in the Bhopal disaster which killed nearly 8,000 people initially and approximately 20,000 - 30,000 people in total.[2][3][4][5]
Contents
Physical properties
Methyl isocyanate (MIC) is a colorless, lachrymatory, flammable liquid.[6] Methyl isocyanate is soluble in water to 6–10 parts per 100 parts, but it reacts with the water (see Reactions).
Manufacture
Methyl isocyanate is usually manufactured by the reaction of monomethylamine and phosgene. For large scale production, it is advantageous to combine these reactants at higher temperature in the gas phase. A mixture of methyl isocyanate and two moles of hydrogen chloride is formed, but N-methylcarbamoyl chloride (MCC) forms as the mixture is condensed, and leaves one mole of hydrogen chloride as a gas.

The methyl isocyanate is obtained by treating the MCC with an amine such as dimethylaniline, pyridine)[7] or by separating it by using distillation techniques.[8]

Methyl isocyanate is also manufactured from N-methylformamide and air. In the latter process, it is immediately consumed in a closed-loop process to make methomyl.[9] Other manufacturing methods have been reported.[10][11]
Reactions
Methyl isocyanate reacts readily with many substances that contain N-H or O-H groups. With water, it forms 1,3-dimethylurea and carbon dioxide with the evolution of heat (325 calories per gram of MIC):

At 25 °C, in excess water, one-half of the MIC is consumed in 9 min.;[12] if the heat is not efficiently removed from the mixture, the rate of the reaction will increase and rapidly cause the MIC to boil. If MIC is in excess, 1,3,5-trimethylbiuret is formed along with carbon dioxide.[6]Alcohols and phenols, which contain an O-H group, react slowly with MIC, but the reaction can be catalyzed by trialkylamines or dialkyltin dicarboxylate.Oximes, hydroxylamines, and enols also react with MIC to form methylcarbamates.[6] These reactions produce the products described below (Uses).

Ammonia, primary, and secondary amines rapidly react with MIC to form substituted ureas. Other N-H compounds, such as amides and ureas, react much more slowly with MIC.[13]
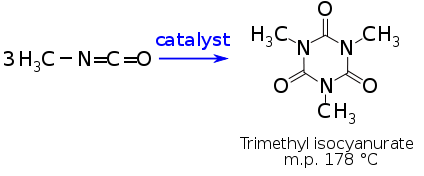
It also reacts with itself to form a trimer or higher molecular weight polymers. In the presence of catalysts, MIC reacts with itself to form a solid trimer, trimethyl isocyanurate, or a higher molecular weight polymer:
Sodium methoxide, triethyl phosphine, ferric chloride, and certain other metal compounds catalyze the formation of the MIC-trimer, while the high-molecular-weight polymer formation is catalyzed by certain trialkylamines. Since the formation of the MIC trimer is exothermic (298 calories per gram of MIC), the reaction can lead to violent boiling of the MIC. The high-molecular-weight polymer hydrolyzes in hot water to form the trimethyl isocyanurate. Since catalytic metal salts can be formed from impurities in commercial grade MIC and steel, this product must not be stored in steel drums or tanks.[6]
Uses
Methyl isocyanate is an intermediate chemical in the production of carbamate pesticides (such as carbaryl, carbofuran, methomyl, and aldicarb). It has also been used in the production of rubbers and adhesives.
Hazards
Methyl isocyanate (MIC) is extremely toxic. The threshold limit value set by the American Conference on Government Industrial Hygienist was 0.02 ppm. MIC can damage by inhalation, ingestion and contact in quantities as low as 0.4 ppm. Damage includes coughing, chest pain, dyspnea, asthma, irritation of the eyes, nose and throat, as well as skin damage. Higher levels of exposure, over 21 ppm, can result in pulmonary or lung edema, emphysema and hemorrhages, bronchial pneumonia and death. Although the odor of methyl isocyanate cannot be detected at 5 ppm by most people, its potent lachrymal properties provide an excellent warning of its presence (at a concentration of 2–4 parts per million (ppm) subject's eyes are irritated, while at 21 ppm, subjects could not tolerate the presence of methyl isocyanate in air).[14]
Proper care must be taken to store methyl isocyanate because of its ease of exothermically polymerizing (see Reactions) and its similar sensitivity to water. Only stainless steel or glass containers may be safely used; the MIC must be stored at temperatures below 40 °C (104 °F) and preferably at 4 °C (39 °F).
The toxic effect of the compound was apparent in the Bhopal disaster, when around 42,000 kilograms (93,000 lb) of methyl isocyanate and other gases were released from the underground reservoirs of Union Carbide India Limited (UCIL) factory, over a populated area on December 3, 1984, immediately killing thousands and leading to the deaths of tens of thousands in subsequent weeks and months.
References
- ^ a b c Lide, David R., ed (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. ISBN 0-8493-0487-3.
- ^ Broughton, Edward (2005). "The Bhopal disaster and its aftermath: a review". Environmental Health 4 (1): 6. doi:10.1186/1476-069X-4-6. PMC 1142333. PMID 15882472. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1142333.
- ^ Eckerman, Ingrid (2001). "Chemical Industry and Public Health — Bhopal as an example". http://www.lakareformiljon.org/images/stories/dokument/2009/bhopal_gas_disaster.pdf.
- ^ Eckerman, Ingrid (2004). The Bhopal Saga - Causes and Consequences of the World's Largest Industrial Disaster. India: Universities Press. ISBN 81-7371-515-7. http://www.eckerman.nu/default.cfm?page=The%20Bhopal%20Saga.
- ^ Rosenberg, Jennifer. "At 1984 - Huge Poison Gas Leak in Bhopal, India". About.com. http://history1900s.about.com/od/1980s/qt/bhopal.htm. Retrieved 2008-07-10.
- ^ a b c d Union Carbide Corporation "Methyl Isocyanate" Product Information Publication, F-41443, November 1967.
- ^ Slocombe R. J. & Hardy E. E., Process of Producing Carbamyl Chlorides U. S. Patent No. 2,480,088, August 23, 1949.
- ^ Merz W, "Procédé et dispositif de préparation d'isocyanates d'alkyle French Patent No. 1,400,863 assigned to Farbenfabriken Bayer AG, Germany 1965.
- ^ Chemical Week, "A fleeting existence for toxic-gas molecules" p. 9, June 12, 1985.
- ^ Giesselmann G., Guenther K., Fuenten W., Methyl Isocyanate,'German Patent No. 2,828,259, January 10, 1980 (cited in Chemical Abstracts 92: 214882n).
- ^ Chemical Week, "A safer method for making carbamates" p. 136, no. 20, 1985b.
- ^ Enrique A. Castro, Roy B. Moodie and Peter J. Sansom (1985). "The kinetics of hydrolysis of methyl and phenyl lsocyanates". J. Chem. Soc., Perkin Trans. 2 1985 (5): 737. doi:10.1039/P29850000737.
- ^ March J. (1985). Advanced Organic Chemistry (3rd ed.). New York: John Wiley & Sons. pp. 802.
- ^ Kimmerle G, and Eben A., "Zur Toxicität von Methylisocyanat und dessen quantitativer Bestimmung in der Luft", Achiv fur Toxikologie, no. 20, 235–241, 1964.
External links
- NIOSH Safety and Health Topic: Isocyanates, from the website of the National Institute for Occupational Safety and Health (NIOSH)
- U.S. National Library of Medicine: Hazardous Substances Databank – Methyl isocyanate
Categories:- Isocyanates
- Monomers
- Bhopal disaster
Wikimedia Foundation. 2010.