- Naringin
-
Naringin 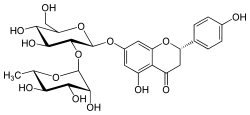 7-[[2-O-(6-Deoxy-α-L-mannopyranosyl)-β-D-
7-[[2-O-(6-Deoxy-α-L-mannopyranosyl)-β-D-
glucopyranosyl]]oxy]-2,3-dihydro-5-hydroxy-
2-(4-hydroxyphenyl)-4H-1-benzopyran-4-oneOther namesNaringin
Naringoside
4',5,7-Trihydroxyflavanone-7-rhamnoglucoside
4',5,7-Trihydroxyflavanone-7-rutinosideIdentifiers CAS number 10236-47-2 PubChem 442428 ChemSpider 4447695 
UNII N7TD9J649B 
Jmol-3D images Image 1 - O=C4c5c(O)cc(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O[C@H]1O[C@@H]([C@H](O)[C@H](O)[C@H]1O)C)cc5O[C@H](c3ccc(O)cc3)C4
- InChI=1S/C27H32O14/c1-10-20(32)22(34)24(36)26(37-10)41-25-23(35)21(33)18(9-28)40-27(25)38-13-6-14(30)19-15(31)8-16(39-17(19)7-13)11-2-4-12(29)5-3-11/h2-7,10,16,18,20-30,32-36H,8-9H2,1H3/t10-,16+,18-,20+,21-,22+,23+,24-,25-,26-,27-/m1/s1

Key: DFPMSGMNTNDNHN-CSIAVLANSA-N
InChI=1/C27H32O14/c1-10-20(32)22(34)24(36)26(37-10)41-25-23(35)21(33)18(9-28)40-27(25)38-13-6-14(30)19-15(31)8-16(39-17(19)7-13)11-2-4-12(29)5-3-11/h2-7,10,16,18,20-30,32-36H,8-9H2,1H3/t10-,16+,18-,20+,21-,22+,23+,24-,25-,26-,27-/m1/s1
Key: DFPMSGMNTNDNHN-CSIAVLANBN
Properties Molecular formula C27H32O14 Molar mass 580.54 g/mol Exact mass 580.179206 Melting point 166 °C
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Naringin is a flavanone glycoside. It is a major flavonoid in grapefruit and gives the grapefruit juice its bitter taste. It is metabolized to the flavanone naringenin in humans. Both naringenin and hesperetin, which are the aglycones of naringin and hesperidin, occur naturally in citrus fruits.
Activity
Naringin exerts a variety of pharmacological effects such as antioxidant activity, blood lipid lowering, anticancer activity, and inhibition of selected drug-metabolizing cytochrome P450 enzymes, including CYP3A4 and CYP1A2, which may result in drug-drug interactions in vivo.[1] Ingestion of naringin and related flavonoids can also affect the intestinal absorption of certain drugs, leading to either an increase or decrease in circulating drug levels. To avoid interference with drug absorption and metabolism, the consumption of citrus (esp. grapefruit) and other juices with medications is contraindicated. [2]
Naringin, followed by rutin, was the most potent flavonoid inhibitor of VEGF release, which causes angiogenesis, out of 21 flavonoids.[3]
Use
When naringin is treated with potassium hydroxide or another strong base, and then catalytically hydrogenated, it becomes a naringin dihydrochalcone, a compound roughly 300-1800 times sweeter than sugar at threshold concentrations[4].
References
- ^ Grapefruit Juice and Medications
- ^ "BBC NEWS, Health, Fruit juice 'could affect drugs'". 2008-08-20. http://newsvote.bbc.co.uk/mpapps/pagetools/print/news.bbc.co.uk/2/hi/health/7572500.stm. Retrieved 2008-08-25.
- ^ Schindler R, Mentlein R (June 2006). "Flavonoids and vitamin E reduce the release of the angiogenic peptide vascular endothelial growth factor from human tumor cells". J. Nutr. 136 (6): 1477–82. PMID 16702307. http://jn.nutrition.org/cgi/content/full/136/6/1477.
- ^ Tomasik, Piotr (2004). Chemical and functional properties of food saccharides. Boca Raton: CRC Press. p. 389. ISBN 0-8493-1486-0.
Flavanones O-methylated flavanones C-methylated flavanones PoriolGlycosides Eriocitrin | Hesperidin | Liquiritin | Naringin | Narirutin | Poncirin | SakuraninAcetylated 8-prenylnaringeninAcetylated glycosides NirurinBond Geometry Glycone Aglycone biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/i Categories:- Flavanone glycosides
- Bitter compounds
- Rutinosides
Wikimedia Foundation. 2010.
