- Organic solar cell
-
An organic photovoltaic cell (OPVC) is a photovoltaic cell that uses organic electronics--a branch of electronics that deals with conductive organic polymers or small organic molecules[1] for light absorption and charge transport.
The plastic itself has low production costs in high volumes. Combined with the flexibility of organic molecules, this makes it potentially lucrative for photovoltaic applications. Molecular engineering (e.g. changing the length and functional group of polymers) can change the energy gap, which allows chemical change in these materials. The optical absorption coefficient of organic molecules is high, so a large amount of light can be absorbed with a small amount of materials. The main disadvantages associated with organic photovoltaic cells are low efficiency, low stability and low strength compared to inorganic photovoltaic cells.
Contents
Organic photovoltaic materials
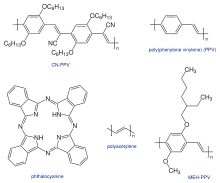
A photovoltaic cell is a specialized semiconductor diode that converts visible light into direct current (DC) electricity. Some photovoltaic cells can also convert infrared (IR) or ultraviolet (UV) radiation into DC. A common characteristic of both the small molecules and polymers (Fig 1) used in photovoltaics is that they all have large conjugated systems. A conjugated system is formed where carbon atoms covalently bond with alternating single and double bonds, in other words these are chemical reactions of hydrocarbons. These hydrocarbons electrons pz orbitals delocalize and form a delocalized bonding π orbital with a π* antibonding orbital. The delocalized π orbital is the highest occupied molecular orbital (HOMO), and the π* orbital is the lowest unoccupied molecular orbital (LUMO). The separation between HOMO and LUMO is considered as the band gap of organic electronic materials. The band gap is typically in the range of 1-4 eV.[2]
When these materials absorb a photon, an excited state is created and confined to a molecule or a region of a polymer chain. The excited state can be regarded as an electron hole pair bound together by electrostatic interactions, i.e. excitons. In photovoltaic cells, excitons are broken up into free electrons-hole pairs by effective fields. The effective field are set up by creating a heterojunction between two dissimilar materials. Effective fields break up excitons by causing the electron to fall from the conduction band of the absorber to the conduction band of the acceptor molecule. It is necessary that the acceptor material has a conduction band edge that is lower than that of the absorber material.[3][4][5][6]
Types of junctions for OPVC
Single layer organic photovoltaic cell
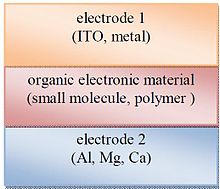
Single layer organic photovoltaic cells are the simplest form among various organic photovoltaic cells. These cells are made by sandwiching a layer of organic electronic materials between two metallic conductors, typically a layer of indium tin oxide (ITO) with high work function and a layer of low work function metal such as Al, Mg and Ca. The basic structure of such a cell is illustrated in Fig 2.
The difference of work function between the two conductors sets up an electric field in the organic layer. When the organic layer absorbs light, electrons will be excited to Lowest Unoccupied Molecular Orbital (LUMO) and leave holes in the Highest Occupied Molecular Orbital (HOMO) forming excitons. The potential created by the different work functions helps to separate the exciton pairs, pulling electrons to the positive electrode (an electrical conductor used to make contact with a nonmetallic part of a circuit) and holes to the negative electrode. The current and voltage resulting from this process can be used to do work. Using electric fields is not the best way to break up excitons, heterojunction based cells which rely on effective fields are more effective.[3][4][5]
Examples
Cells with phthalocyanine (Fig 1) as organic layer were investigated at the early stage. As early as 1958, Kearns et al. reported the photovoltaic effector the creation of voltage of a cell based on magnesium phthalocyanine a macrocyclic compound having an alternating nitrogen atom-carbon atom ring structure (MgPh), which had a photovoltage of 200mV.[7] Ghosh et al. investigated the Al/MgPh/Ag cell, and obtained photovoltaic efficiency of 0.01% under illumination at 690 nm.[8]
Conjugated polymers were also used in this type of photovoltaic cell. Weinberger et al. used polyacetylene (Fig 1) as the organic layer, Al and graphite as electrodes to fabricate a cell, which had an open circuit voltage of 0.3 V and a charge collection efficiency of 0.3%.[9] Glenis et al. reported a Al/poly(3-nethyl-thiophene)/Pt cell had an external quantum yield of 0.17%, an open circuit voltage of 0.4V and a fill factor of 0.3.[10] Karg et al. fabricated an ITO/PPV/Al cell, showing an open circuit voltage of 1V and a power conversion efficiency of 0.1% under white-light illumination.[11]
Problems
In practice, single layer organic photovoltaic cells of this type do not work well. They have low quantum efficiencies (<1%) and low power conversion efficiencies (<0.1%). A major problem with them is the electric field resulting from the difference between the two conductive electrodes is seldom sufficient to break up the photogenerated excitons. Often the electrons recombine with the holes rather than reach the electrode. To deal with this problem, the multilayer organic photovoltaic cells were developed.
Bilayer organic photovoltaic cells
This type of organic photovoltaic cell contains two different layers in between the conductive electrodes (Fig 3). These two layers of materials have differences in electron affinity and ionization energy, therefore electrostatic forces are generated at the interface between the two layers. The materials are chosen properly to make the differences large enough, so these local electric fields are strong, which may break up the excitons much more efficiently than the single layer photovoltaic cells do. The layer with higher electron affinity and ionization potential is the electron acceptor, and the other layer is the electron donor. This structure is also called planar donor-acceptor heterojunctions.[3][4][5][6]
Examples
C60 has high electron affinity, making it a good material as electron acceptor in photovoltaic cells of this type. Sariciftci et al. fabricated a C60/MEH-PPV double layer cell, which had a relatively high fill factor of 0.48 and a power conversion efficiency of 0.04% under monochromatic illumination.[12] For PPV/C60 cells, Halls et al. reported a monochromatic external quantum efficiency of 9%, a power conversion efficiency of 1% and a fill factor of 0.48.[13]
Perylene derivatives are a group of organic molecules with high electron affinity and chemical stability. Ching W. Tang deposited a layer of copper phthalcocyanine as electron donor and perylene tetracarboxylic derivative as electron acceptor, fabricating a cell with a fill factor as high as 0.65 and a power conversion efficiency of 1% under simulated AM2 illumination.[14] Halls et al. fabricated a cell with a layer of bis(phenethylimido) perylene over a layer of PPV as the electron donor. This cell had peak external quantum efficiency of 6% and power conversion efficiency of 1% under monochromatic illumination, and the fill factor is up to 0.6.[15]
Problems
The diffusion length of excitons in organic electronic materials is typically on the order of 10 nm. In order for most excitons to diffuse to the interface of layers and break up into carriers, the layer thickness should also be in the same range with the diffusion length. However, typically a polymer layer needs a thickness of at least 100 nm to absorb enough light. At such a large thickness, only a small fraction of the excitons can reach the heterojunction interface. To address this problem, a new type of heterojunction photovoltaic cells is designed, which is the dispersed heterojunction photovoltaic cells.
Bulk heterojunction photovoltaic cells
In this type of photovoltaic cell, the electron donor and acceptor are mixed together, forming a polymer blend (Fig 4). If the length scale of the blend is similar with the exciton diffusion length, most of the excitons generated in either material may reach the interface, where excitons break efficiently. Electrons move to the acceptor domains then were carried through the device and collected by one electrode, and holes were pulled in the opposite direction and collected at the other side.[4][5][7]
Examples
C60 and its derivatives are also used as electron acceptor in the dispersed heterojunction photovoltaic cells. Yu et al. fabricated a cell with the blend of MEH-PPV and a methano-functionalized C60 derivative as the heterojunction, ITO and Ca as the electrodes.[16] This cell showed a quantum efficiency of 29% and a power conversion efficiency of 2.9% under monochromatic illumination. Later they replaced MEH-PPV with P3OT, which obtained a cell with a quantum yield of 45% under a 10V reverse bias.[17][18]
Polymer/polymer blends are also used in dispersed heterojunction photovoltaic cells. Halls et al. used a blend of CN-PPV and MEH-PPV, fabricated a cell with Al and ITO as the electrodes, whose peak monochromatic power conversion efficiency is 1% and fill factor is 0.38.[19][20]
Dye sensitized photovoltaic cells can also be considered as important ones of this type.
Graded Heterojunction photovoltaic cells
In this type of photovoltaic cell, the electron donor and acceptor are mixed together, like in the bulk heterojunction, but in such as way that the gradient is gradual. This architecture combines the short electron travel distance in the dispersed heterojunction with the advantage of the charge gradient of the bilayer technology[21].
Examples
Holmes et al. fabricated a cell with a blend of CuPc and C60. The cell showed a quantum efficiency of 50% and a power conversion efficiency of 2.1% using 100 mW/cm2 simulated AM1.5G solar illumination for a graded heterojunction. [22]
Current challenges and recent progress
Difficulties associated with organic photovoltaic cells include their low quantum efficiency (~3%) in comparison with inorganic photovoltaic devices; due largely to the large band gap of organic materials. Instabilities against oxidation and reduction, recrystallization and temperature variations can also lead to device degradation and decreased performance over time. This occurs to different extents for devices with different compositions, and is an area into which active research is taking place.[23]
Other important factors include the exciton diffusion length; charge separation and charge collection; and charge transport and mobility, which are affected by the presence of impurities.
Effect of film morphology
As described in section 2.3, dispersed heterojunction of donor-acceptor organic materials have high quantum efficiency compared to the planar hetero-junction, because it is more likely for an exciton to find an interface within its diffusion length. Film morphology can also have a drastic effect on the quantum efficiency of the device. Rough surfaces and presence of voids can increase the series resistance and also the chance of short circuiting. Film morphology and as a result quantum efficiency can be improved by annealing of a device after covering it by ~1000Å thick metal cathode. Metal film on top of the organic film applies stresses on the organic film, which helps to prevent the morphological relaxation in the organic film. This gives more densely packed films while at the same time allows the formation of phase-separated interpenetrating donor-acceptor interface inside the bulk of organic thin film.[24]
Controlled growth heterojunction
Charge separation occurs at the donor acceptor interface. Whilst traveling to the electrode, a charge can become trapped and/or recombine in a disordered interpenetrating organic material, resulting in decreased device efficiency. Controlled growth of the heterojunction provides better control over positions of the donor-acceptor materials, resulting in much greater power efficiency (ratio of output power to input power) than that of planar and highly disoriented hetero-junctions (as shown in Fig 5(a), (b)). Thus, the choice of suitable processing parameters in order to better control the structure and film morphology is highly desirable.[25]
Progress in growth techniques
Mostly organic films for photovoltaic applications are deposited by spin coating and vapor-phase deposition. However each method has certain draw backs, spin coating technique can coat larger surface areas with high speed but the use of solvent for one layer can degrade the already existing polymer layer. Another problem is related with the patterning of the substrate for device as spin-coating results in coating the entire substrate with a single material.
Vacuum thermal evaporation
Another deposition technique is "Vacuum thermal evaporation" (VTE) which involves the heating of an organic material in vacuum. The substrate is placed several centimeters away from the source so that evaporated material may be directly deposited onto the substrate, as shown in Fig 6(a). This method is useful for depositing many layers of different materials without chemical interaction between different layers. However, there are sometimes problems with film-thickness uniformity and uniform doping over large-area substrates. In addition, the materials that deposit on the wall of the chamber can contaminate later depositions. This is "line of sight" technique also can create holes in the film due to shadowing, which causes an increase in the device series-resistance and short circuit.[26]
Organic vapor phase deposition
Organic thin film grown from "Organic vapor phase deposition" (OVPD) is proven to give better control on the structure and morphology of the film than vacuum thermal evaporation. The process involves evaporation of the organic material over a substrate in the presence of an inert carrier gas. Resulting film morphology can be changed by changing the gas flow rate and the source temperature. Uniform film can be grown by reducing the carrier gas pressure, which will increase the velocity and mean free path of the gas, and as a result boundary layer thickness decreases. Cells produced by OVPD do not have issues related with contaminations from the flakes coming out of the walls of the chamber, as the walls are warm and do not allow molecules to stick to and produce a film upon them.
Another advantage over VTE is the uniformity in evaporation rate. This occurs because the carrier gas becomes saturated with the vapors of the organic material coming out of the source and then moves towards the cooled substrate, Fig6(b). Depending on the growth parameters (temperature of the source, base pressure and flux of the carrier gas) the deposited film can be crystalline or amorphous in nature. Devices fabricated using OVPD show a higher short-circuit current density than that of devices made using VTE. An extra layer of donor-acceptor hetero-junction at the top of the cell may block excitons, whilst allowing conduction of electron; resulting in improved cell efficiency.[26]
Organic solar ink
Plextronics Plexcore PV 2000 organic solar ink is able to deliver higher performance in fluorescent lighting conditions in comparison to amorphous silicon solar cells, and said to have a 30% to 40% increase in indoor power density in comparison to the standard organic solar technology. [27]
Plextronics has also developed a manufacturing method that allows for low-temperature processing of OPV. While previous industry standard techniques required a glass substrate to be annealed at temperatures at or above 110° C, this method enables annealing at less than 65° C. It is expected to reduce manufacturing costs as it may enable the use of less expensive substrates, especially once the process is transferred to flexible substrates such as plastic.
See also
References
- ^ Pulfry L.D., Photovoltaic Power Generation,(New York : Van Nostrand Reinhold Co., 1978)
- ^ Rivers N.P.Leading edge research in solar energy,(2007)
- ^ a b c McGehee D.G., Topinka M.A. Nature Mater. 5, 675-676 (2006)
- ^ a b c d Nelson J. Current Opinion in Solid State and Materials Science 6, 87-95 (2002)
- ^ a b c d Halls J.J.M., Friend R.H. In: Archer M.D., Hill R.D. editors, Clean electricity from photovoltaics, London: Imperial College Press, 377-445 (2001)
- ^ a b H. Hoppe and N. S. Sariciftci, J. Mater. Res. 19, 1924-1945 (2004)
- ^ a b Kearns D., Calvin M. J.Chem.Phys. 29, 950-951 (1958)
- ^ Ghosh A.K. et al. J.Appl.Phys. 45,230-236 (1974)
- ^ Weinberger B.R. et al. Synth.Metals 4, 187-197 (1982)
- ^ Glenis S. et al. Thin Solid Films, 139, 221-231 (1986)
- ^ Karg S. et al. Snth.Metals 54, 427-433 (1993)
- ^ Sariciftci N.S. et al. Appl.Phys.Lett. 62, 585-587 (1993)
- ^ Halls J.J.M. et al. Appl. Phys. Lett. 68,3120-3122 (1996)
- ^ Tang C.W. Appl.Phys.Lett. 48, 183-185 (1986)
- ^ Halls J.J.M. et al. Synth. Metals 85, 1307-1308(1997)
- ^ Yu G. et al. Science 270, 1789-1791 (1995)
- ^ Yu G. et al. Adv.Mat. 10, 1431-1434 (1998)
- ^ kaneko M. photocatalysis: Science and technology,(1999)
- ^ Halls J.J.M. et al. Nature 376, 498-500 (1995)
- ^ Seraphin B.O. Topics in applied physicsP; solar energy conversion: solid-state physics aspects(vol.31), (1979)
- ^ "Graded Heterojunction". http://www.license.umn.edu/Products/Organic-Photovoltaic-Solar-Cells-using-Graded-Heterojunction-Technology__Z09174.aspx.
- ^ Holmes, Russel; Richa Pandey (November/December 2010). "Organic Photovoltaic Cells Based on Continuously Graded Donor–Acceptor Heterojunctions". IEEE JOURNAL OF SELECTED TOPICS IN QUANTUM ELECTRONICS 16 (6): 7. http://ieeexplore.ieee.org/stamp/stamp.jsp?tp=&arnumber=5491035. Retrieved 16 August 2011.
- ^ Li B. et al. Solar Energy Materials & Solar Cells 90, 549 - 537 (1982)
- ^ Peumans P. et al. Nature, 425, 158-162(2003)
- ^ Yang F. et al. Nature Mater., 4, 37-41 (2005)
- ^ a b Forrest S.R. Nature, 428, 911-918 (2004)
- ^ http://www.pv-tech.org/news/_a/plextronics_announces_developments_in_organic_photovoltaics/
Further reading
- Electronic Processes in Organic Crystals and Polymers, 2 ed. by Martin Pope and Charles E. Swenberg, Oxford University Press (1999), ISBN 0195129636
- Organic Photovoltaics by Christoph Brabec, Vladimir Dyakonov, Jürgen Parisi and Niyazi Serdar Sariciftci (eds.), Springer Verlag (Berlin, 2003), ISBN 3-540-00405-X
- Organic Photovoltaics: Mechanisms, Materials, and Devices (Optical Engineering) by Sam-Shajing Sun and Niyazi Serdar Sariciftci (eds.), CRC Press (2005), ISBN 0-8247-5963-X
- Handbook of Organic Electronics and Photonics (3-Volume Set) by Hari Singh Nalwa, American Scientific Publishers. (2008), ISBN 1-58883-095-0
- Progress in Photovoltaics: Research and Applications, Volume 18, Issue 5, Solar Cell Efficiency Tables (version 36), Wiley 2010
Photovoltaics Concepts - Photoelectric effect
- Photovoltaics
- History of photovoltaics
- Timeline of solar cells
- Solar insolation
- Solar constant
- Solar cell efficiency
- Third generation photovoltaic cell
- Solar cell research
- Quantum efficiency of a solar cell
- Cadmium telluride
- Thermophotovoltaic
- Polycrystalline silicon photovoltaics
- Thermodynamic efficiency limit
- Sun-free photovoltaics
- Polarizing organic photovoltaics

Photovoltaic system Solar cells- Solar cell
- Solar panel
- Thin film solar cell
- Polymer solar cell
- Nanocrystal solar cell
- Organic solar cell
- Quantum dot solar cell
- Hybrid solar cell
- Plasmonic solar cell
- Carbon nanotubes in photovoltaics
- Dye-sensitized solar cell
- Cadmium telluride photovoltaics
- Copper indium gallium selenide solar cells
- Multijunction photovoltaic cell
- Printed solar panel
System components- Solar charge controller
- Solar inverter
- Solar micro-inverter
- Solar cable
- Solar combiner box
- Photovoltaic mounting system
- Maximum power point tracker
- Solar tracker
- Solar shingles
- Solar mirror
System concepts- Perturb and observe method
- Incremental conductance method
- Constant voltage method
- Fill factor
- Concentrated photovoltaics
- Photovoltaic thermal hybrid solar collector
- Space-based solar power
- Watt-peak
Applications Appliances- Solar-powered refrigerator
- Solar air conditioning
- Solar lamp
- Solar charger
- Solar backpack
- Solar tree
- Solar-powered pump
- Solar-powered watch
- Solar Tuki
- Photovoltaic keyboard
- Solar road stud
- Solar cell phone charger
- Solar notebook
- Solar powered calculator
- Solar powered fountain
- Solar powered radio
- Solar powered flashlight
- Solar fan
- Solar street light
- Solar traffic light
Land transport- Solar vehicle
- Solar car
- Solar roadway
- Solar golf cart
- The Quiet Achiever
- Sunmobile
Air transport- Electric aircraft
- Mauro Solar Riser
- Solar panels on spacecraft
- Solar-Powered Aircraft Developments Solar One
- Gossamer Penguin
- Qinetiq Zephyr
- Solar Challenger
Water transportSolar vehicle racing- Solar car racing
- List of solar car teams
- Solar challenge
- Solar Cup
- Blue Sky Solar Racing
- Frisian Solar Challenge
- UC Solar Team
- Solar Splash
- South African Solar Challenge
- Tour de Sol
- World Solar Challenge
- Hunt-Winston School Solar Car Challenge
- North American Solar Challenge
- Victorian Model Solar Vehicle Challenge
Generation systems - Solar Energy Generating Systems
- Stand-alone photovoltaic power system
- Grid-connected photovoltaic power system
- Rooftop photovoltaic power station
- Topaz Solar Farm
- Solar Ark
- Solar Umbrella house
- Erlasee Solar Park
- Guadarranque solar power plant
- Pocking Solar Park
- Copper Mountain Solar Facility
- Wyandot Solar Facility
- Köthen Solar Park
- Building-integrated photovoltaics
- Moura Photovoltaic Power Station
- Nevada Solar One
- Beneixama photovoltaic power plant
- Gottelborn Solar Park
- Darro Solar Park
- Olmedilla Photovoltaic Park
- Blythe Photovoltaic Power Plant
- Strasskirchen Solar Park
- Puertollano Photovoltaic Park
- Alamosa photovoltaic power plant
By countryList of countries by photovoltaics productionPV companies  Category ·
Category ·  CommonsCategories:
CommonsCategories:- Organic solar cells
- Solar cells
Wikimedia Foundation. 2010.