- Degarelix
-
Degarelix 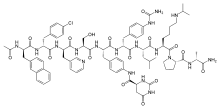
Clinical data AHFS/Drugs.com Multum Consumer Information MedlinePlus a609022 Licence data EMA:Link, US FDA:link Pregnancy cat. N/A Legal status ℞-only (US) Prescription only Routes Subcutaneous injection Pharmacokinetic data Bioavailability 30-40% Protein binding ~90% Metabolism Subject to common peptidic degradation during passage through the hepato-biliary system; not a substrate for the human CYP450 system Half-life 23-61 days Excretion ~20-30% in the urine, ~70-80% in the faeces Identifiers CAS number 214766-78-6 
ATC code L02BX02 PubChem CID 16186010 DrugBank DB06699 UNII SX0XJI3A11 
KEGG D08901 
ChEMBL CHEMBL264089 
Chemical data Formula C84H107ClN18O18 Mol. mass 1692.311180 g/mol  (what is this?) (verify)
(what is this?) (verify)Degarelix (INN) or degarelix acetate (USAN) (tradename Firmagon) is a hormonal therapy used in the treatment of prostate cancer. During development it was known as FE200486.
Testosterone is a male hormone that promotes growth of many prostate tumours and therefore reducing circulating testosterone to very low (castration) levels is often the treatment goal in the management of men with advanced prostate cancer. Degarelix has an immediate onset of action, binding to gonadotropin-releasing hormone (GnRH) receptors in the pituitary gland and blocking their interaction with GnRH. This induces a fast and profound reduction in luteinising hormone (LH), follicle-stimulating hormone (FSH) and in turn, testosterone suppression.[1]
Contents
Status
On 24 December 2008, the Food and Drug Administration (FDA) approved degarelix for the treatment of patients with advanced prostate cancer in the USA.[2] It was subsequently approved by the European Commission at the recommendation of the European Medicines Agency (EMEA) on February 17, 2009 for use in adult male patients with advanced, hormone-dependent prostate cancer. Ferring Pharmaceuticals markets the drug under the name Firmagon.
Mode of action
GnRH antagonists (receptor blockers) such as degarelix are a new type of hormonal therapy for prostate cancer. These agents are synthetic peptide derivatives of the natural GnRH decapeptide – a hormone that is made by neurons in the hypothalamus. GnRH antagonists compete with natural GnRH for binding to GnRH receptors in the pituitary gland. This reversible blinding blocks the release of LH and FSH from the pituitary. The reduction in LH subsequently leads to a rapid and sustained suppression of testosterone release from the testes and subsequently reduces the size and growth of the prostate cancer. This in turn results in a reduction in prostate-specific antigen (PSA) levels in the patient's blood. Measuring PSA levels is a way to monitor how patients with prostate cancer are responding to treatment.
Unlike the GnRH agonists, which cause an initial stimulation of the hypothalamic-pituitary-gonadal axis (HPGA), leading to a surge in testosterone levels, and under certain circumstances, a flare-up of the tumour, GnRH antagonists do not cause a surge in testosterone or clinical flare.[3] Clinical flare is a phenomenon that occurs in patients with advanced disease, which can precipitate a range of clinical symptoms such as bone pain, ureteral obstruction, and spinal cord compression. Drug agencies have issued boxed warnings regarding this phenomenon in the prescribing information for GnRH agonists. As testosterone surge does not occur with GnRH antagonists, there is no need for patients to receive an antiandrogen as flare protection during prostate cancer treatment. GnRH agonists also induce an increase in testosterone levels after each reinjection of the drug – a phenomenon that does not occur with GnRH antagonists such as degarelix.
GnRH antagonists have an immediate onset of action leading to a fast and profound suppression of testosterone and are therefore especially valuable in the treatment of patients with prostate cancer where fast control of disease is needed.
Clinical effectiveness
A Phase III, randomised, 12 month clinical trial (CS21) in prostate cancer[4] compared androgen deprivation with one of two doses of degarelix or the GnRH agonist, leuprolide. Both degarelix doses were at least as effective as leuprolide at suppressing testosterone to castration levels (≤0.5 ng/mL) from Day 28 to study end (Day 364). Testosterone levels were suppressed significantly faster with degarelix than with leuprolide, with degarelix uniformly achieving castration levels by Day 3 of treatment which was not seen in the leuprolide group. There were no testosterone surges with degarelix compared with surges in 81% of those who received leuprolide. Degarelix resulted in a faster reduction in PSA levels compared with leuprolide indicating faster control of the prostate cancer. Recent results also suggest that degarelix therapy may result in longer control of prostate cancer compared with leuprolide.[5]
Side effects
As with all hormonal therapies, degarelix is commonly associated with hormonal side effects such as hot flushes and weight gain.[4][6][7] Due to its mode of administration (subcutaneous injection), degarelix is also associated with injection-site reactions such as injection-site pain, erythema or swelling. Injection-site reactions are usually mild or moderate in intensity and occur predominantly after the first dose, decreasing in frequency thereafter.[4]
FSH receptors in other solid tumors
FSH receptors are selectively expressed on the luminal surface of the blood vessels of a wide range of tumors.[8] There may be a potential role for suppression of FSH or FSH receptors. This work is in early stages. It is thought that FSH receptors are important in tumor angiogenesis by signalling via two pathways, one involving VEGF, and a Gq/11 mechanism that activates VEGFR-2 independently of VEGF.[8]
References
- ^ Princivalle M, Broqua P, White R, et al (March 2007). Rapid suppression of plasma testosterone levels and tumor growth in the dunning rat model treated with degarelix, a new gonadotropin-releasing hormone antagonist. J. Pharmacol. Exp. Ther. 320: 1113-8.
- ^ PR Newswire. FDA approves Ferring Pharmaceuticals' Degarelix (generic name) for the treatment of advanced prostate cancer. PR Newswire, Europe Ltd 2008 [cited 2009 Mar 2]; Available from here
- ^ Van Poppel H, Nilsson S (June 2008). Testosterone surge: rationale for gonadotropin-releasing hormone blockers? Urology 71: 1001-6.
- ^ a b c Klotz L, Boccon-Gibod L, Shore ND, et al (December 2008). The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 102: 1531-8.
- ^ Schröder FH, Boccon-Gibod L, Tombal B, et al (March 2009) Degarelix versus leuprolide in patients with prostate cancer: effect in metastatic patients as assessed by serum alkaline phosphatase. European Association of Urology (EAU) Annual congress 17–21 March 2009, Stockholm, Sweden. Abstract 40.
- ^ Gittelman M, Pommerville PJ, Persson BE, et al (November 2008). A 1-year, open label, randomized phase II dose finding study of degarelix for the treatment of prostate cancer in North America. J. Urol. 180: 1986-92.
- ^ Van Poppel H, Tombal B, de la Rosette JJ, et al (October 2008). Degarelix: a novel gonadotropin-releasing hormone (GnRH) receptor blocker--results from a 1-yr, multicentre, randomised, phase 2 dosage-finding study in the treatment of prostate cancer. Eur. Urol. 54: 805-13.
- ^ a b Radu, A.; Pichon, C.; Camparo, P.; Antoine, M.; Allory, Y.; Couvelard, A.; Fromont, G. L.; Hai, M. T. V. et al. (2010). "Expression of Follicle-Stimulating Hormone Receptor in Tumor Blood Vessels". New England Journal of Medicine 363 (17): 1621–1630. doi:10.1056/NEJMoa1001283. PMID 20961245.
External links
Gonadotropins and GnRH (G03G) Gonadotropin
preparationsAgonistAntigonadotropinGnRH Categories:- GnRH antagonists
Wikimedia Foundation. 2010.
