- Oleoylethanolamide
-
Oleoylethanolamide 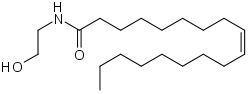 (Z)-N-(2-hydroxyethyl)octadec-9-enamide
(Z)-N-(2-hydroxyethyl)octadec-9-enamideIdentifiers CAS number 111-58-0 
PubChem 5283454 Jmol-3D images Image 1 - CCCCCCCC\C=C/CCCCCCCC(=O)NCCO
Properties Molecular formula C20H39NO2 Molar mass 325.53 Appearance White solid Melting point 59-60°C
Solubility in water soluble in ethanol and DMSO  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Oleoylethanolamine (OEA) is an endogenous peroxisome proliferator-activated receptor alpha (PPAR-α) agonist. It is a naturally occurring ethanolamide lipid that regulates feeding and body weight in vertebrates ranging from mice to pythons.[1][2][3]
OEA is the monounsaturated analogue of the endocannabinoid anandamide, but unlike anandamide it acts independently of the cannabinoid pathway, regulating PPAR-α activity to stimulate lipolysis.[4]
OEA is produced by the small intestine following feeding in two steps. First an N-acyl transferase (NAT) activity joins the free amino terminus of phosphatidylethanolamine (PE) to the oleoyl group (one variety of acyl group) derived from sn-1-oleoyl-phosphatidylcholine, which contains the fatty acid oleic acid at the sn-1 position. (illustration). This produces an N-acylphosphatidylethanolamine, which is then split (hydrolyzed) by N-acyl phosphatidylethanolamine-specific phospholipase D (NAPE-PLD) into phosphatidic acid and OEA.
OEA has recently been demonstrated to bind to the novel cannabinoid receptor GPR119.[5] OEA has been suggested to be the receptor's endogenous ligand.[6]
References
- ^ Gaetani S, Oveisi F, Piomelli D (2003). "Modulation of meal pattern in the rat by the anorexic lipid mediator oleoylethanolamine". Neuropsychopharmacology 28 (7): 1311–6. doi:10.1038/sj.npp.1300166. PMID 12700681.
- ^ Lo Verme J, Gaetani S, Fu J, Oveisi F, Burton K, Piomelli D (2005). "Regulation of food intake by oleoylethanolamine". Cell. Mol. Life Sci. 62 (6): 708–16. doi:10.1007/s00018-004-4494-0. PMID 15770421.
- ^ Giuseppe Astarita, Bryan C. Rourke, Johnnie B. Andersen, Jin Fu, Janet H. Kim, Albert F. Bennett, James W. Hicks, and Daniele Piomelli (2005-12-22). "Postprandial increase of oleoylethanolamine mobilization in small intestine of the Burmese python (Python molurus)". Am J Physiol Regul Integr Comp Physiol 290 (5): R1407–R1412. doi:10.1152/ajpregu.00664.2005. PMID 16373434. http://ajpregu.physiology.org/cgi/content/full/290/5/R1407.
- ^ Gaetani S, Kaye WH, Cuomo V, Piomelli D (September 2008). "Role of endocannabinoids and their analogues in obesity and eating disorders". Eat Weight Disord 13 (3): e42–8. PMID 19011363. http://www.kurtis.it/abs/index.cfm?id_articolo_numero=4959.
- ^ Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, Jackson HC, Procter MJ, Rasamison CM, Tang-Christensen M, Widdowson PS, Williams GM, Reynet C. (2006). "Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents.". Cell Metab. 3 (3): 167–175. doi:10.1016/j.cmet.2006.02.004. PMID 16517404.
- ^ Brown AJ. (2007). "Novel cannabinoid receptors.". Br J Pharmacol. 152 (5): 567–575. doi:10.1038/sj.bjp.0707481. PMC 2190013. PMID 17906678. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2190013.
External links

This pharmacology-related article is a stub. You can help Wikipedia by expanding it.
