- List of cocaine analogues
-
Cocaine with it's numerical substitution positions.

This is a list of cocaine analogues. A cocaine analogue retains 3β-benzoyloxy or similar functionality unlike phenyltropanes. Cocaine analogues proper consist of the nine following classes of compounds:[1]
- stereoisomers of cocaine
- 3β-phenyl ring substituted analogues
- 2β-substituted analogues
- N-modified analogues of cocaine
- 3β-carbamoyl analogues
- 3β-alkyl-3-benzyl tropanes
- 6/7-subtituted cocaines
- 6-alkyl-3-benzyl tropanes
- piperidine homologues of cocaine
The term may also be loosely used to refer to drugs manufactured from cocaine or having their basis as a total synthesis of cocaine, but modified to alter their effect & QSAR. These include both intracellular sodium channel blocker anesthetics and phenyltropane stimulant dopamine reuptake inhibitor ligands (such as certain piperidines). Many of the stimulant created analogues are of a class of C-2 substituted benzoyloxytropanes.
Contents
Analogs sensu stricto
Cocaine Stereoisomers
Stereoisomer IC50 (nM) R-cocaine 102 edit] Benzoyl- branch cleavage substitutions (excluding the exhaustive phenyl- group) & active transesterification metabolites of cocaine - Cocaethylene
- 2'-Acetoxycocaine
- 4'-Fluorococaine[2]
- Benzoylthiomethylecgonine[3]
- Salicylmethylecgonine
- Methylvanillylecgonine[4]
- Isothiocyanatobenzoylecgonine methyl ester
Miscellaneous stimulant analogues
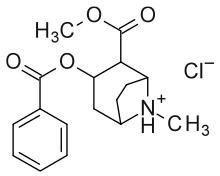 Alternate two-dimensional molecular diagram of cocaine; shown specifically as a protonated, NH+, hydrochloride, and disregarding 3D stereochemistry
Alternate two-dimensional molecular diagram of cocaine; shown specifically as a protonated, NH+, hydrochloride, and disregarding 3D stereochemistry
Analogues with both stimulant & local anesthetic effects
- Dimethocaine/Larocaine (DMC)
- 3-(p-Fluorobenzoyloxy)tropane (30% stimulant potency of cocaine & equipotent as an anaesthetic)
See some of Clarke's contributions
Quinuclidine Analogues
- Butyltolylquinuclidine
Phenyltropanes series (excluding all other benzoyl- branch substitutions)
See: List of phenyltropanes
Piperidine Analogues
Organochloride Analogues
GBR Analogs
- GBR-12909
- GBR-12935
Benztropine Analogs
- Benztropine
- Difluoropine (more selective as a DARI than cocaine. Also an anticholinergic & antihistamine)
- AHN 1-055 Same structure as for benztropine but 4',4'-bisfluorinated.
- GA 103 N-phenylpropyl bis-4-fluorobenztropine
- JHW 007[5] N-(n-butyl)-3α-[bis(4'-fluorophenyl)methoxy]-tropane
"Difluoropine" is not a phenyltropane but actually belongs to the benztropine family of DRIs.
In certain respects these are important because they share SAR overlap with GBR 12909 and related analogs.SARs have shown that 4',4'-difluorination is an excellent way to boost DAT activity of benztropine, and gives excellent selectivity over the SERT and the NET.[6][7]
Furthermore, replacing the N-Me with, e.g. n-phenylpropyl helps to bring muscarinic activity down to something that is the same as DRI affinty.[6]
This is remarkable considering unmodified (native) benztropine is 60 times more active as an anticholinergic than as a dopaminergic.[6]
M1 receptor considerations aside, analogues of this benztropine class still won't substitute for cocaine, and have no propensity to elevate locomotor activity.
Phenethylamines
Many phenethylamines are dopamine releasers, however, certain drugs of the family inhibit dopamine reuptake & transport which may be loosely classed as cocaine analogs. Dependent upon their specific configurations.
Local anesthetics (not usually CNS stimulants)
Note: Certain of the local anesthetics still have residual DRI properties,[8] although not normally ones that are easily available. These are expected to be more cardiotoxic than phenyltropanes. For example, dimethocaine has behavioral stimulant effects (and therefore not here listed below) if a dose of it is taken that is 10 times the amount of cocaine. Dimethocaine is equipotent to cocaine in terms of its anesthetic equivalency.[8]
- Amylocaine
- Articaine
- Benzocaine
- Bupivacaine (Marcaine, Sensorcaine, Vivacaine)
- Butacaine
- Carticaine
- Chloroprocaine (Nesacaine)
- Cinchocaine/Dibucaine (Nupercaine)
- Cyclomethycaine (Surfacaine, Topocaine)
- Etidocaine
- Hexylcaine (Cyclaine, Osmocaine)
- Levobupivacaine (Chirocaine)
- Lidocaine/Lignocaine (Xylocaine)
- Mepivacaine (Carbocaine, Polocaine)
- Meprylcaine/Oracaine
- Metabutoxycaine (Primacaine)
- Piperocaine (Metycaine)
- Prilocaine
- Propoxycaine/Ravocaine
- Procaine/Novocaine (Novocain)
- Proparacaine/Alcaine
- Risocaine
- Ropivacaine
- Tetracaine/Amethocaine (Pontocaine)
- Trimecaine
Analogues for other purposes
See also
Common analogues to prototypical DRAs:
-
- Substituted amphetamines
- Substituted cathinones
- Substituted phenethylamines
- Substituted methylenedioxyphenethylamines
References
- ^ Chemisty, Design, and Structure-Activity Relationship of Cocaine Antagonists. Satendra Singh et al. Chem. Rev. 2000, 100. 925-1024. Final paragraph pg. 45
- ^ Gatley SJ, Yu DW, Fowler JS, MacGregor RR, Schlyer DJ, Dewey SL, Wolf AP, Martin T, Shea CE, Volkow ND (March 1994). "Studies with differentially labeled [11C]cocaine, [11C]norcocaine, [11C]benzoylecgonine, and [11C]- and 4'-[18F]fluorococaine to probe the extent to which [11C]cocaine metabolites contribute to PET images of the baboon brain". Journal of Neurochemistry 62 (3): 1154–62. doi:10.1046/j.1471-4159.1994.62031154.x. PMID 8113802.
- ^ Benzoylthio-. cocaine, analogue substitution
- ^ Smith, R. Martin; Poquette, Michael A.; Smith, Paula J., "Hydroxymethoxybenzoylmethylecgonines: New metabolites of cocaine from human urine." Journal of Analytical Toxicology 1984, 8(1), pp.29-34
- ^ Tanda G, Newman A, Ebbs AL, Tronci V, Green J, Tallarida RJ, Katz JL.Combinations of Cocaine with other Dopamine Uptake Inhibitors: Assessment of Additivity. J Pharmacol Exp Ther. 2009 May 29. PMID 19483071
- ^ a b c Rothman RB, Baumann MH, Prisinzano TE, Newman AH. Dopamine transport inhibitors based on GBR12909 and benztropine as potential medications to treat cocaine addiction. Biochem Pharmacol. 2008 Jan 1;75(1):2-16. doi:10.1016/j.bcp.2007.08.007 PMID 17897630
- ^ Runyon SP, Carroll FI. Dopamine transporter ligands: recent developments and therapeutic potential. Curr Top Med Chem. 2006;6(17):1825-43. doi:10.2174/156802606778249775 PMID 17017960
- ^ a b Wilcox, K.M., Kimmel, H.L., Lindsey, K.P., Votaw, J.R., Goodman, M.M., Howell, L.L. In vivo comparison of the reinforcing and dopamine transporter effects of local anesthetics in rhesus monkeys. Synapse, 58: 220-228, 2005. PDF
External links
- U. S. Provisional Patent Application listing examples of compounds which are tropanes for prospective use in research
- Article on cocaine analogue research
Categories:- Cocaine
- Dopamine reuptake inhibitors
- Stimulants
- Local anesthetics
- Tropanes
Wikimedia Foundation. 2010.
Look at other dictionaries:
Cocaine — For other uses, see Cocaine (disambiguation). Cocaine … Wikipedia
List of misconceptions about illegal drugs — Many urban legends and misconceptions about classified drugs have been created and circulated among children and the general public, with varying degrees of veracity. These are commonly repeated by organizations which oppose all classified drug… … Wikipedia
(+)-CPCA — Systematic (IUPAC) name methyl (3R,4S) 4 (4 chlorophenyl) 1 methylpiperidine 3 carboxylate Clinical data Pregnancy cat … Wikipedia
Troparil — Systematic (IUPAC) name methyl (1R,2S,3S,5S) 8 methyl 3 phenyl 8 azabicyclo[3.2.1]octane 2 carboxylate Clinical data Pregnancy cat … Wikipedia
WIN 35428 — Systematic (IUPAC) name methyl (1R,2S,3S,5S) 3 (4 fluorophenyl) 8 methyl 8 azabicyclo[3.2.1]octane 2 carboxylate Clinical data Pr … Wikipedia
RTI-51 — Systematic (IUPAC) name methyl (1R,2S,3S,5S) 3 (4 bromophenyl) 8 methyl 8 azabicyclo[3.2.1]octane 2 carboxylate Clinical data Pregnancy cat … Wikipedia
(-)-2β-(1,2,4-Oxadiazol-5-methyl)-3β-phenyltropane — Systematic (IUPAC) name (1R,2S,3S,5S) 8 methyl 2 (1,2,4 oxadiazol 5 methyl) 3 phenyl 8 azabicyclo[3.2.1]octane Clinical data Pregna … Wikipedia
N-(2'-Fluoroethyl-)-3β-(4'-chlorophenyl)-2β-(3'-phenylisoxazol-5'-yl)nortropane — Systematic (IUPAC) name (1R,2S,3S,5S) 3 (4 chlorophenyl) 8 (2 fluoroethyl) 2 (3 phenylisoxazol 5 yl) 8 azabicyclo[3.2.1]octane … Wikipedia
N-(3'-Fluoropropyl-)-3β-(4'-chlorophenyl)-2β-(3'-phenylisoxazol-5'-yl)nortropane — Systematic (IUPAC) name (1R,2S,3S,5S) 3 (4 chlorophenyl) 8 (3 fluoropropyl) 2 (3 phenylisoxazol 5 yl) 8 azabicyclo[3.2.1]octane … Wikipedia
N,O-Dimethyl-4-(2-naphthyl)piperidine-3-carboxylate — Systematic (IUPAC) name methyl (3S,4S) 1 methyl 4 (2 naphthyl)piperidine 3 carboxylate Clinical data Pr … Wikipedia



