- CRYAB
-
Crystallin, alpha B 
Rendering based on PDB 2KLR.Available structures PDB 2KLR, 2WJ7, 3L1G Identifiers Symbols CRYAB; CRYA2; CTPP2; HSPB5 External IDs OMIM: 123590 MGI: 88516 HomoloGene: 68209 GeneCards: CRYAB Gene Gene Ontology Molecular function • structural constituent of eye lens
• protein binding
• protein homodimerization activity
• unfolded protein bindingCellular component • nucleus
• cytoplasmBiological process • protein folding
• anti-apoptosis
• muscle contraction
• response to heat
• negative regulation of intracellular transport
• protein homooligomerizationSources: Amigo / QuickGO RNA expression pattern 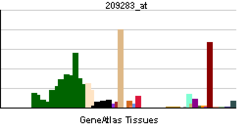
More reference expression data Orthologs Species Human Mouse Entrez 1410 12955 Ensembl ENSG00000109846 ENSMUSG00000032060 UniProt P02511 Q52L78 RefSeq (mRNA) NM_001885.1 NM_009964.2 RefSeq (protein) NP_001876.1 NP_034094.1 Location (UCSC) Chr 11:
111.78 – 111.79 MbChr 9:
50.56 – 50.56 MbPubMed search [1] [2] Alpha-crystallin B chain is a protein that in humans is encoded by the CRYAB gene.[1]
Crystallins are separated into two classes: taxon-specific, or enzyme, and ubiquitous. The latter class constitutes the major proteins of vertebrate eye lens and maintains the transparency and refractive index of the lens. Since lens central fiber cells lose their nuclei during development, these crystallins are made and then retained throughout life, making them extremely stable proteins. Mammalian lens crystallins are divided into alpha, beta, and gamma families; beta and gamma crystallins are also considered as a superfamily. Alpha and beta families are further divided into acidic and basic groups.
Seven protein regions exist in crystallins: four homologous motifs, a connecting peptide, and N- and C-terminal extensions. Alpha crystallins are composed of two gene products: alpha-A and alpha-B, for acidic and basic, respectively. Alpha crystallins can be induced by heat shock and are members of the small heat shock protein (sHSP also known as the HSP20) family. They act as molecular chaperones although they do not renature proteins and release them in the fashion of a true chaperone; instead they hold them in large soluble aggregates. Post-translational modifications decrease the ability to chaperone.
These heterogeneous aggregates consist of 30-40 subunits; the alpha-A and alpha-B subunits have a 3:1 ratio, respectively. Two additional functions of alpha crystallins are an autokinase activity and participation in the intracellular architecture. Alpha-A and alpha-B gene products are differentially expressed; alpha-A is preferentially restricted to the lens and alpha-B is expressed widely in many tissues and organs. Elevated expression of alpha-B crystallin occurs in many neurological diseases; a missense mutation cosegregated in a family with a desmin-related myopathy.[2]
Contents
Interactions
CRYAB has been shown to interact with CRYBB2,[3] Hsp27,[4][3] HSPB2,[5] CRYGC,[3] PSMA3[6] and CRYAA.[3]
See also
References
- ^ Jeanpierre C, Austruy E, Delattre O, Jones C, Junien C (Mar 1993). "Subregional physical mapping of an alpha B-crystallin sequence and of a new expressed sequence D11S877E to human 11q". Mamm Genome 4 (2): 104–8. doi:10.1007/BF00290434. PMID 8431633.
- ^ "Entrez Gene: CRYAB crystallin, alpha B". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=1410.
- ^ a b c d Fu, Ling; Liang Jack J-N (Feb. 2002). "Detection of protein-protein interactions among lens crystallins in a mammalian two-hybrid system assay". J. Biol. Chem. (United States) 277 (6): 4255–60. doi:10.1074/jbc.M110027200. ISSN 0021-9258. PMID 11700327.
- ^ Kato, K; Shinohara H, Goto S, Inaguma Y, Morishita R, Asano T (Apr. 1992). "Copurification of small heat shock protein with alpha B crystallin from human skeletal muscle". J. Biol. Chem. (UNITED STATES) 267 (11): 7718–25. ISSN 0021-9258. PMID 1560006.
- ^ Sugiyama, Y; Suzuki A, Kishikawa M, Akutsu R, Hirose T, Waye M M, Tsui S K, Yoshida S, Ohno S (Jan. 2000). "Muscle develops a specific form of small heat shock protein complex composed of MKBP/HSPB2 and HSPB3 during myogenic differentiation". J. Biol. Chem. (UNITED STATES) 275 (2): 1095–104. doi:10.1074/jbc.275.2.1095. ISSN 0021-9258. PMID 10625651.
- ^ Boelens, W C; Croes Y, de Jong W W (Jan. 2001). "Interaction between alphaB-crystallin and the human 20S proteasomal subunit C8/alpha7". Biochim. Biophys. Acta (Netherlands) 1544 (1–2): 311–9. ISSN 0006-3002. PMID 11341940.
External links
Further reading
- Derham BK, Harding JJ (1999). "Alpha-crystallin as a molecular chaperone". Progress in retinal and eye research 18 (4): 463–509. doi:10.1016/S1350-9462(98)00030-5. PMID 10217480.
- Calinisan V, Gravem D, Chen RP et al. (2006). "New insights into potential functions for the protein 4.1 superfamily of proteins in kidney epithelium". Front. Biosci. 11: 1646–66. doi:10.2741/1911. PMID 16368544.
Opsin (retinylidene protein) Crystallin Other M: EYE
anat(g/a/p)/phys/devp/prot
noco/cong/tumr, epon
proc, drug(S1A/1E/1F/1L)
Categories:- Human proteins
- Chromosome 11 gene stubs
Wikimedia Foundation. 2010.
