- Diethyl ether peroxide
-
Diethyl ether hydroperoxide 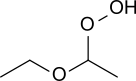
Identifiers CAS number 18321-53-4 ChemSpider 19985446 
Jmol-3D images Image 1
Image 2- CCOC(OO)C
CC(OCC)OO
Properties Molecular formula C4H10O3 Molar mass 106.12 g/mol Density 1.005 g/cm³ Boiling point 62 - 64 °C at 18.7 hPa (reduced pressure)
Hazards Main hazards Explosive NFPA 704  ether peroxide (verify) (what is:
ether peroxide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Diethyl ether peroxides are a class of organic peroxides that slowly form in diethyl ether upon storage under air, light, or in the presence of metal by autoxidation.
Contents
Diethyl ether hydroperoxide
Diethyl ether hydroperoxide (CH3-CH2-O-CH(OOH)-CH3) is a colorless liquid of low viscosity with a pleasant smell. Upon heating it weakly deflagrates, resulting in a fog of acetic acid and water. Diethyl ether hydroperoxide decomposes in the presence of sodium hydroxide and Fe2+-containing salts.
Diethyl ether peroxide
Diethyl ether peroxide, also known as ethylidene peroxide, (-CH(CH3)OO-)n is a polymerization product of diethyl ether hydroperoxide. It is a colorless oily liquid that is an extremely brisant and friction sensitive explosive material. Amounts of less than 5 milligrams can damage chemical apparatuses.[who?] The dangerous properties of ether peroxides are the reason that diethyl ether and other peroxide forming ethers like tetrahydrofuran (THF) or ethylene glycol dimethyl ether (1,2-dimethoxyethane) are avoided in industrial processes.

Tests
Diethyl ether peroxides can be detected with potassium iodide (KI) solution or potassium iodide / starch paper. A positive test results in the formation of iodine (I2) that causes a pink color of the ether phase or a dark bluish spot on the paper strip.
Degradation
Ether peroxides can be destroyed by disproportionation to acetaldehyde with Fe2+ or Mn2+ ions or with triphenylphosphine (PPh3). The resulting aldehyde has to be removed to prevent a rapid back-formation of peroxides.
References
- A. Rieche, R. Meister, Modellversuche zur Autoxidation der Äther, Angewandte Chemie 49(5):106 (1936) (German)
Categories:- Organic peroxides
- CCOC(OO)C
Wikimedia Foundation. 2010.

