- Nitrogen trichloride
-
Nitrogen trichloride 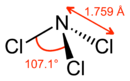
 Other namesTrichloramine
Other namesTrichloramine
Agene
Nitrogen(III) chloride
Trichloroazane
Trichlorine nitrideIdentifiers CAS number 10025-85-1 PubChem 61437 ChemSpider 55361 
ChEBI CHEBI:37382 
RTECS number QW974000 Jmol-3D images Image 1 - ClN(Cl)Cl
Properties Molar mass 120.365 g/mol Appearance yellow oily liquid Density 1.635 g/mL Melting point -40 °C, 233 K, -40 °F
Boiling point 71 °C, 344 K, 160 °F
Solubility in water Immiscible
slowly decomposesSolubility soluble in benzene, chloroform, CCl4, CS2, PCl3 Structure Crystal structure rhombohedral (below -40 °C) Molecular shape trigonal pyramidal Dipole moment 0.6 D Thermochemistry Std enthalpy of
formation ΔfHo298+232 kJ/mol Standard molar
entropy So298? J.K−1.mol−1 Hazards EU classification not listed NFPA 704 Autoignition
temperature93 °C Related compounds Other anions Nitrogen trifluoride
Nitrogen tribromide
Nitrogen triiodideOther cations Phosphorus trichloride
Arsenic trichlorideRelated chloramines Chloramine
DichloramineRelated compounds Nitrosyl chloride  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Nitrogen trichloride, also known as trichloramine, is the chemical compound with the formula NCl3. This yellow, oily, pungent-smelling liquid is most commonly encountered as a byproduct of chemical reactions between ammonia-derivatives and chlorine (for example, in swimming pools between disinfecting chlorine and urea in urine from bathers).
In pure form, NCl3 is highly reactive. Nitrogen trichloride can form in small amounts when public water supplies are disinfected with monochloramine, and at given levels it can irritate mucous membranes.[1] Nitrogen trichloride was trademarked as Agene and used to artificially bleach and age flour. It has the same effect as that of tear gas, but has never been used as such.[2]
Contents
Preparation and structure
The compound is prepared by treatment of ammonium salts, such as ammonium nitrate with chlorine:
- 4 NH3 + 3 Cl2 → NCl3 + 3 NH4Cl
Intermediates in this conversion include chloramine and dichloramine, NH2Cl and NHCl2, respectively.
Like ammonia, NCl3 is a pyramidal molecule. The N-Cl distances are 1.76 Å, and the Cl-N-Cl angles are 107°.[3] The Pauling electronegativities are very similar for nitrogen (3.04) and chlorine (3.16).
Reactions
The nitrogen in NCl3 is often considered to have the -3 oxidation state and the chlorine atoms are considered to be in the +1 oxidation state. Most of its reactivity is consistent with this description.
Nitrogen trichloride is hydrolyzed by hot water to release ammonia and hypochlorous acid.
- NCl3 + 3 H2O → NH3 + 3 HOCl
Safety
Nitrogen trichloride is a dangerous explosive, being sensitive to light, heat, and organic compounds. Pierre Louis Dulong first prepared it in 1812, and lost two fingers and an eye in two separate explosions. An explosion from NCl3 blinded Sir Humphry Davy temporarily, inducing him to hire Michael Faraday as a co-worker. In 2006, Belgian researchers reported a possible link between NCl3 and rising numbers of childhood asthma cases, in what they call the pool chlorine hypothesis, as an alternative to the hygiene hypothesis with a closer causal link.[4]
References
- ^ National Institute for Occupational Safety and Health. (2008, August). NIOSH eNews, 6(4). Retrieved August 27, 2008, from http://www.cdc.gov/niosh/enews/enewsV6N4.html
- ^ George Clifford White: The handbook of chlorination and alternative disinfectants. 4th Edition, Wiley, 1999, ISBN 9780471292074, p. 322
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Bernard A, Carbonnelle S, de Burbure C, Michel O, Nickmilder M (2006). "Chlorinated pool attendance, atopy, and the risk of asthma during childhood". Environmental Health Perspectives 114 (10): 1567–1573. doi:10.1289/ehp.8461. PMC 1626429. PMID 17035144. http://www.ehponline.org/members/2006/8461/8461.pdf.
Further reading
- Jander, J. (1976). Adv. Inorg. Chem. Radiochem. 19: 2.
- P. Kovacic, M. K. Lowery, K. W. Field (1970). "Chemistry of N-bromamines and N-chloramines". Chemical Reviews 70 (6): 639. doi:10.1021/cr60268a002.
- Hartl, H.;, Schoner, J.; Jander, J.; Schulz, H. (1975). "Structure of Solide Nitrogen-Trichloride (-125°C)". Zeitschrift für Anorganische und Allgemeine Chemie 413 (1): 61–71. doi:10.1002/zaac.19754130108.
- Cazzoli, G.; Favero, P. G.; Dalborgo, A. (1974). "Molecular-Structure, Nuclear-Quadruple Coupling-Constant and Dipole-Moment of Nitrogen Trichloride from Microwave Spectroscopy". Journal of Molecular Spectroscopy 50 (1–3): 82. doi:10.1016/0022-2852(74)90219-7.
- Bayersdo, L.; Engelhar, U., Fischer, J.; Hohne, K.; Jander, J. (1969). "Nitrogen-chlorine compounds: Infrared spectra and Raman spectra of nitrogen trichloride". Zeitschrift für anorganische und allgemeine Chemie 366 (3–4): 169–. doi:10.1002/zaac.19693660308.
External links
Categories:- Inorganic amines
- Chlorides
- Nitrogen halides
- Inorganic chlorine compounds
Wikimedia Foundation. 2010.

