- DART ion source
-
 A capsule being analyzed is held in the sample chamber between the DART ion source (right) and the spectrometer inlet (cone on left).
A capsule being analyzed is held in the sample chamber between the DART ion source (right) and the spectrometer inlet (cone on left).
DART (Direct Analysis in Real Time) is an atmospheric pressure ion source that instantaneously ionizes gases, liquids and solids in open air under ambient conditions.[1] It was developed in 2005 by Laramee and Cody and is now marketed commercially by JEOL and IonSense.[2] It was among the first ambient ionization techniques not requiring sample preparation, so solid and liquid materials can be analyzed by mass spectrometry in their native state. Ionization can take place directly on the sample surface, such as, currency bills, tablets, bodily fluids (blood, saliva and urine), glass, plant leaves, fruits & vegetables and even clothing. Liquids are analyzed by dipping an object (such as a glass rod) into the liquid sample and then presenting it to the DART ion source. Vapors are introduced directly into the DART gas stream.
Contents
Principle of operation
Ionization process
The ionization process involves an interaction between the analyte molecule (S) and electronically excited atoms or vibronically excited molecules (metastable species – M*):[citation needed]
Upon collision between the excited gas molecule (M*) and the surface of the sample, an energy transfer takes place, from the excited gas molecule (M*) to the neutral analyte molecule (S). This causes an electron to be released from the analyte molecule, producing a radical cation. The molecular cation is then ejected from the sampling surface and travels to the mass analyzer along with the gas stream (typically N2 or Ne). The process presented in the above equation is called Penning ionization. For this ionization process to take place, the energy of the excited state gas molecule must be higher than the ionization potential of the neutral molecule.
When He is used as the carrier gas, the ionization process occurs by the following mechanism: First an excited state He atom collides with an atmospheric pressure water molecule and ionizes it:
The ionized water molecule then undergoes several reactions with other neutral water molecules resulting in the formation of a protonated water cluster:
The water cluster then interacts with the analyte molecule (S) generating a protonated molecule.
DART can also operate in the negative ion mode by which negatively charged species are formed. The negative ion formation process is under current discussion and investigation.
Formation of metastable species
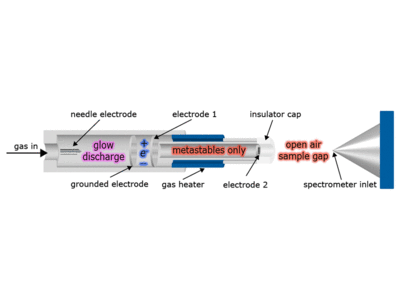
As the gas (N2, Ne or He) enters the ion source, an electric potential in the range of +1 to +5 kV is applied. This generates a glow discharge containing ionized gas, electrons and excited state atoms/molecules (metastable species). A potential of 100 V applied to the electrostatic lenses removes charged particles from the gas stream and only excited state species flow to the third chamber. The gas stream in the third chamber can be heated from RT to 250 oC. Heating is optional but may be necessary depending on the surface or chemical being analyzed. An insulator cap at the terminal end of the ion source protects the operator from harm.
The excited-state species can interact directly with the sample which can be a solid, liquid or gas to desorb and ionize the analyte.
The distance between the ion source and the inlet of the mass spectrometer is 5 to 25 mm. The ions formed are directed to the mass spectrometer inlet by both the gas flow and a slight vacuum in the spectrometer inlet. Although optimum geometries exist for specific applications, the exact positioning, distance and angle of DART ion source with respect to the sample surface and the mass spectrometer inlet are not critical.
Source to analyzer interface
Ions entering the mass spectrometer first go through a source - to - analyzer interface, which was designed in order to minimize spectrometer contamination. The ions are directed to the ion guide through orifice 1 and 2 by applying a slight potential difference between them: orifice 1 - 30V and orifice 2 - 5V. It is clear from the diagram that the space between the two orifices is not horizontal but rather diagonal. Species containing charge (ions) are attracted to the second orifice, but neutral molecules travel in a straight pathway and thus get trapped in that region. The contamination is then removed by the pump.
Mass spectra
DART produces relatively simple mass spectra, dominated by protonated molecules [M+H]+ in positive-ion mode, or deprotonated molecules [M-H]- in negative-ion mode. Depending on the nature of the molecule, other species may be formed, such as M+. from polynuclear aromatic hydrocarbons. Fragmentation may occasionally be observed for some molecules. Multiple-charge ions and alkali metal cation aducts are never observed, but addition of ammonia or other "dopants" to the DART gas stream can be used to form single-charge adducts such as [M+NH4]+ or [M+Cl]- for compounds that would not readily form molecular ions or protonated molecules. For example, the explosives nitroglycerin and HMX do not form [M-H]-,but readily form [M+Cl]- if chloride is present.
Applications
DART can be applied to a wide range of applications, such as, the fragrance industry, pharmaceutical industry, foods and spices, forensic science and health.
In the fragrance industry, the deposition and release of a fragrance on surfaces such as, fabric and hair is often studied. Use of DART compared to traditional methods minimizes sample amount, sample preparation, eliminates extraction steps, decreases limit of detection and analysis time.[3]
In the pharmaceutical industry, there is a growing international problem, which is the production of counterfeit drugs.[4][5] Some countries in which this occurs are United Kingdom, China, Russia, Argentina, Nigeria and India. Dart can detect active ingredients in medicine in a tablet form; there is no need for sample preparation such as, crushing or extracting.
DART was used to directly analyze a red pepper pod in three different places: the membrane (white part holding the seeds), the seeds and the flesh of the pepper. The analyte of interest was capsaicin, a natural ingredient of a red pepper pod that is responsible for the burning sensation when eating chilies. The spectrum obtained revealed that the highest concentration of capsaicin is in the membrane. DART has recently been used in the study of genus Allium plants, e.g., to identify the lachrymatory compound, syn-propanethial S-oxide, C3H6OS, in onion, Allium cepa, a previously unknown lachrymatory compound, syn-butanethial S-oxide, C4H8OS, in Allium siculum,[6] and 2-propenesulfenic acid, an isomer of propanethial S-oxide, which is the very short-lived precursor to allicin from cutting garlic, Allium sativum.[7]
See also
- Desorption electrospray ionization
- Electric glow discharge
- Atmospheric pressure chemical ionization
- Desorption atmospheric pressure photoionization
References
- ^ R.B. Cody, J.A. Laramée, H.D. Durst (2005). "Versatile New Ion Source for the Analysis of Materials in Open Air under Ambient Conditions". Anal. Chem. 77 (8): 2297–2302. doi:10.1021/ac050162j. PMID 15828760.
- ^ 2. “Direct Analysis in Real Time (DARTtm) Mass Spectrometry” Cody, R. B.; Laramée, J. A.; Nilles, J.M.; Durst, H. D. JEOL News; 2005
- ^ O.P. Haefliger, N. Jeckelmann (2007). "Direct mass spectrometric analysis of flavors and fragrances in real applications using DART". Rapid Commun. Mass Spectrom. 21 (8): 1361–1366. doi:10.1002/rcm.2969. PMID 17348088.
- ^ "Bad Medicine". CBC News. December 11, 2005. http://www.cbc.ca/correspondent/feature_051211.html.[dead link]
- ^ "Counterfeit medicines". World Health Organization. November 14, 2006. http://www.who.int/mediacentre/factsheets/fs275/en.
- ^ Kubec R, Cody RB, Dane AJ, Musah RA, Schraml J, Vattekkatte A, Block E (2010). "Applications of DART Mass Spectrometry in Allium Chemistry. (Z)-Butanethial S-Oxide and 1-Butenyl Thiosulfinates and their S-(E)-1-Butenylcysteine S-Oxide Precursor from Allium siculum". Journal of Agricultural and Food Chemistry 58 (2): 1121–1128. doi:10.1021/jf903733e. PMID 20047275.
- ^ Block E, Dane AJ, Thomas S, Cody RB (2010). "Applications of Direct Analysis in Real Time–Mass Spectrometry (DART-MS) in Allium Chemistry. 2-Propenesulfenic and 2-Propenesulfinic Acids, Diallyl Trisulfane 'S'-Oxide and Other Reactive Sulfur Compounds from Crushed Garlic and Other Alliums". Journal of Agricultural and Food Chemistry 58 (8): 4617–4625. doi:10.1021/jf1000106. PMID 20225897.
Patents
- Robert B. Cody and James A. Laramee, “Method for atmospheric pressure ionization” U.S. Patent 6,949,741 issued September 27, 2005. (Priority date: April 2003).
- James A. Laramee and Robert B. Cody “Method for Atmospheric Pressure Analyte Ionization” U.S. Patent 7,112,785 issued September 26, 2006.
Mass spectrometry Ion source Mass analyzer Detector MS combination Fragmentation Categories:- Ion source
- Measuring instruments
Wikimedia Foundation. 2010.




![H_3O^+ + nH_2O \to \left[(H_2O\right)_{n}H]^{+}](2/b32f28e20ade261e5f90be8a1672cbcc.png)
![\left[(H_2O\right)_nH]^{+} + S \to SH^+ + nH_2O](e/ebe5dbae2ee0d87ee7849ff5936ecaa9.png)